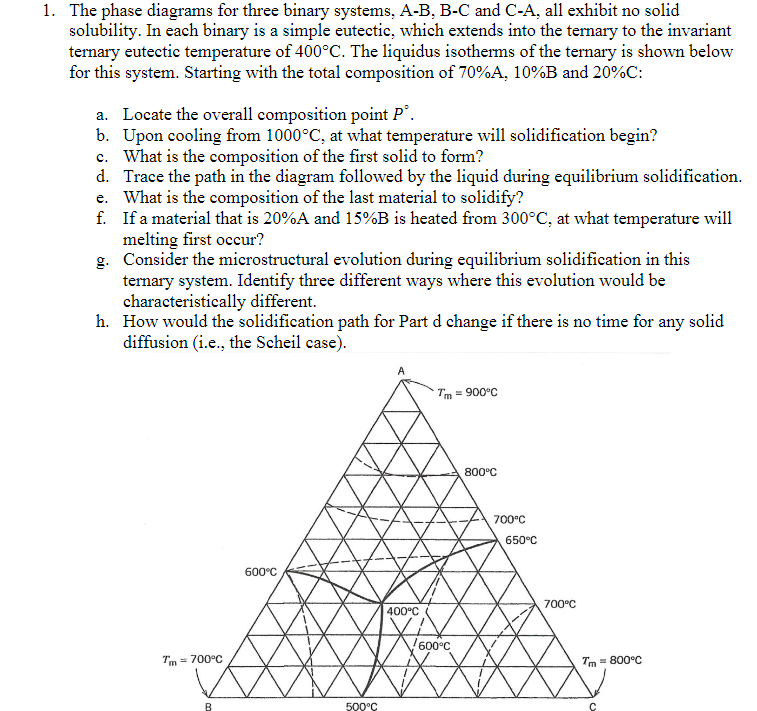
Question: The phase diagrams for three binary systems, A - B , B - C and C - A , all exhibit no solid solubility. In
The phase diagrams for three binary systems, AB BC and CA all exhibit no solid solubility. In each binary is a simple eutectic, which extends into the ternary to the invariant ternary eutectic temperature of The liquidus isotherms of the ternary is shown below for this system. Starting with the total composition of and :
a Locate the overall composition point
b Upon cooling from at what temperature will solidification begin?
c What is the composition of the first solid to form?
d Trace the path in the diagram followed by the liquid during equilibrium solidification.
e What is the composition of the last material to solidify?
f If a material that is A and is heated from at what temperature will melting first occur?
g Consider the microstructural evolution during equilibrium solidification in this ternary system. Identify three different ways where this evolution would be characteristically different.
h How would the solidification path for Part d change if there is no time for any solid diffusion ie the Scheil case
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


