Question: the potential between Cu and the unknown is .98 (some of the pictures may be unneccesary, but wanted to provide all details) Part C: Determine

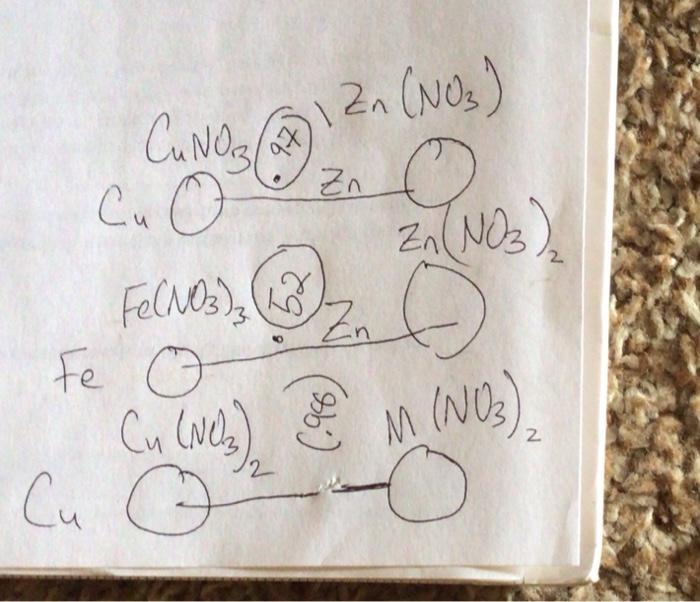
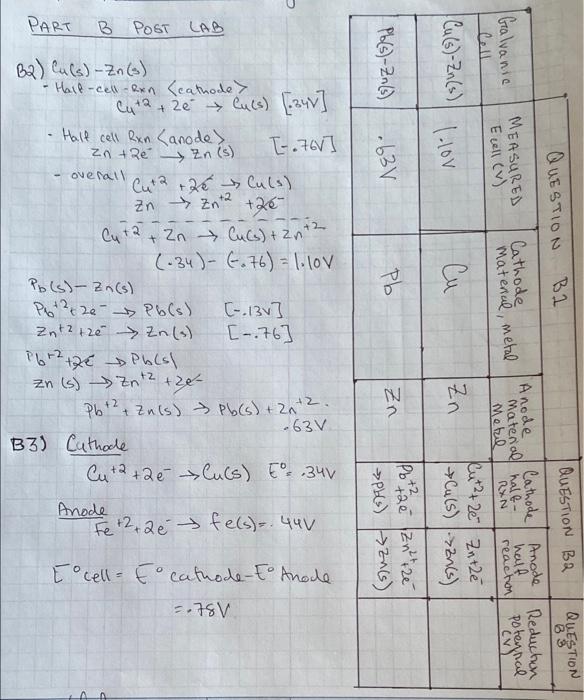
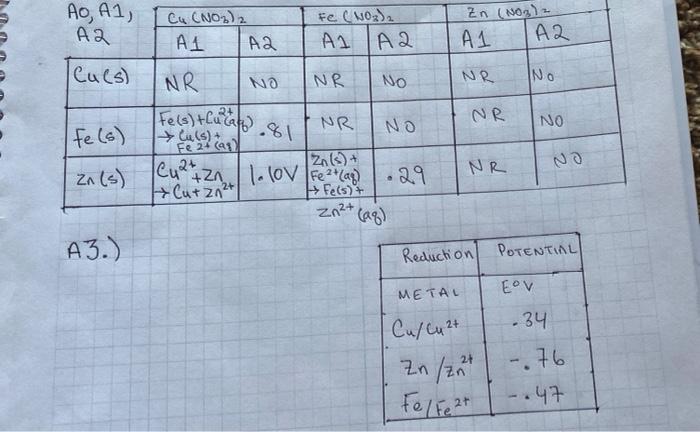
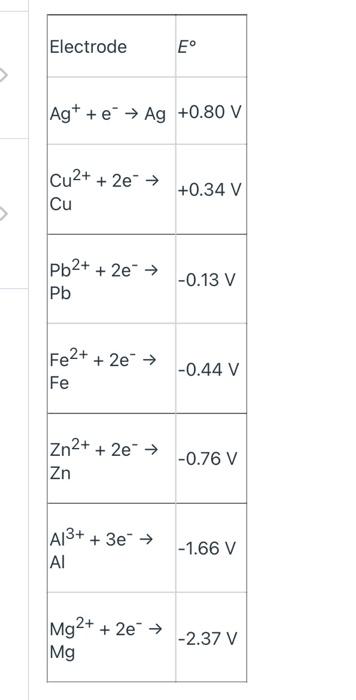
Part C: Determine the E for a Voltaic Cell Using Cu and an Unknown Metal C1. Use equation (5) from the Lab 10 - Background to solve for E anode of the unknown metal, keeping in mind that the Cu is the cathode for the cell when the Ecell is positive. Show all work. C2. Compare the E anode of the unknown to the values listed in Table 10-1 (shown above) and state: "The unknown metal is Cu NO 31 Fe(NO3)3 Fe O Cu (Nels) 97 Zn (NO3) O Zn(NO3) 2NDS ( Zn Zn M (NO3) PART B POST LAB B2) Cu (s)-Zn(s) - Half-cell-Rxn (cathode> Half cell Rxn
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


