Question: The potential energy curves for two nitrogen atoms approaching (solid line) and two neon atoms approaching (dashed line) is shown below. Which of following conclusions
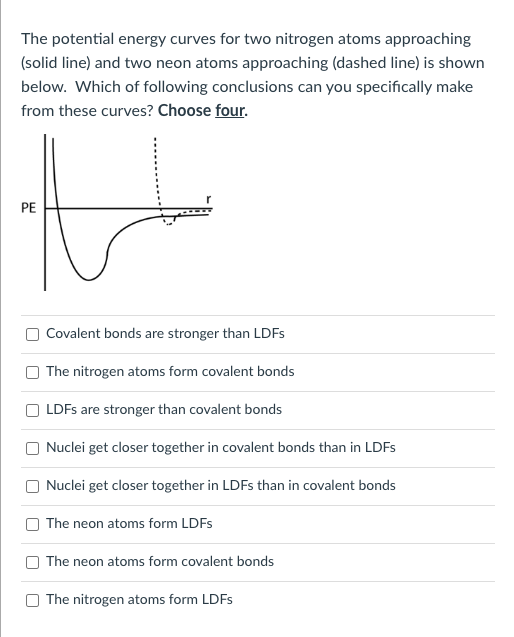
The potential energy curves for two nitrogen atoms approaching (solid line) and two neon atoms approaching (dashed line) is shown below. Which of following conclusions can you specifically make from these curves? Choose four. Covalent bonds are stronger than LDFs The nitrogen atoms form covalent bonds LDFs are stronger than covalent bonds Nuclei get closer together in covalent bonds than in LDFs Nuclei get closer together in LDFs than in covalent bonds The neon atoms form LDFs The neon atoms form covalent bonds The nitrogen atoms form LDFs
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


