Question: The process is shown in the figure below. Hydrogen is added to the gas recycle stream to make the ratio of Hz to CHa 1
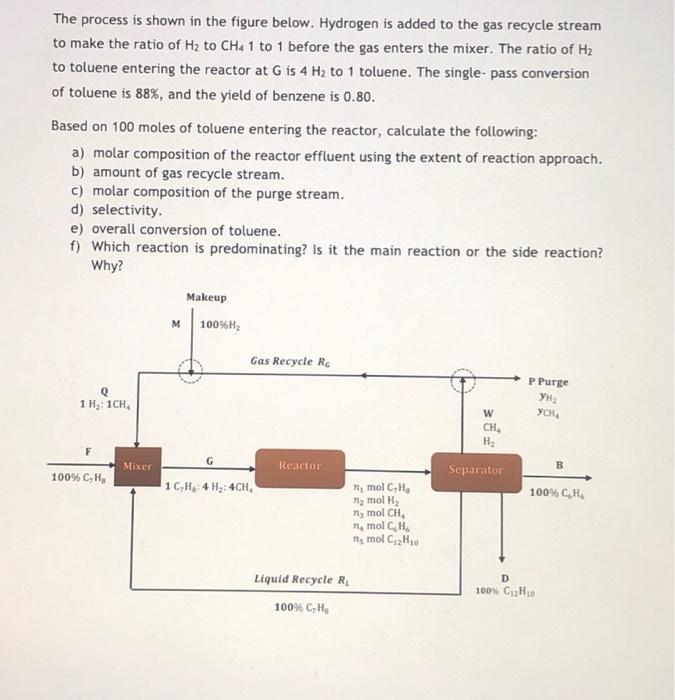
The process is shown in the figure below. Hydrogen is added to the gas recycle stream to make the ratio of Hz to CHa 1 to 1 before the gas enters the mixer. The ratio of H2 to toluene entering the reactor at G is 4 Hz to 1 toluene. The single-pass conversion of toluene is 88%, and the yield of benzene is 0.80. Based on 100 moles of toluene entering the reactor, calculate the following: a) molar composition of the reactor effluent using the extent of reaction approach. b) amount of gas recycle stream. c) molar composition of the purge stream. d) selectivity e) overall conversion of toluene. f) Which reaction is predominating? Is it the main reaction or the side reaction? Why? Makeup M 100%H2 Gas Recycle RC Q 1 H: 1CH P Purge YCH w CH H Mixer Reactor B 100% CH Separator 1C,H, 4 H: 4CH 100% CH ny mol C,H, ng mol H2 Plymol CH na mol CH ms mol C2H10 D Liquid Recycle R. 100% CH. 100% C2H20
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


