Question: The rate constant for this zero-order reaction is 0.0270 M-s-1 at 300 C. A products How long (in seconds) would it take for the concentration
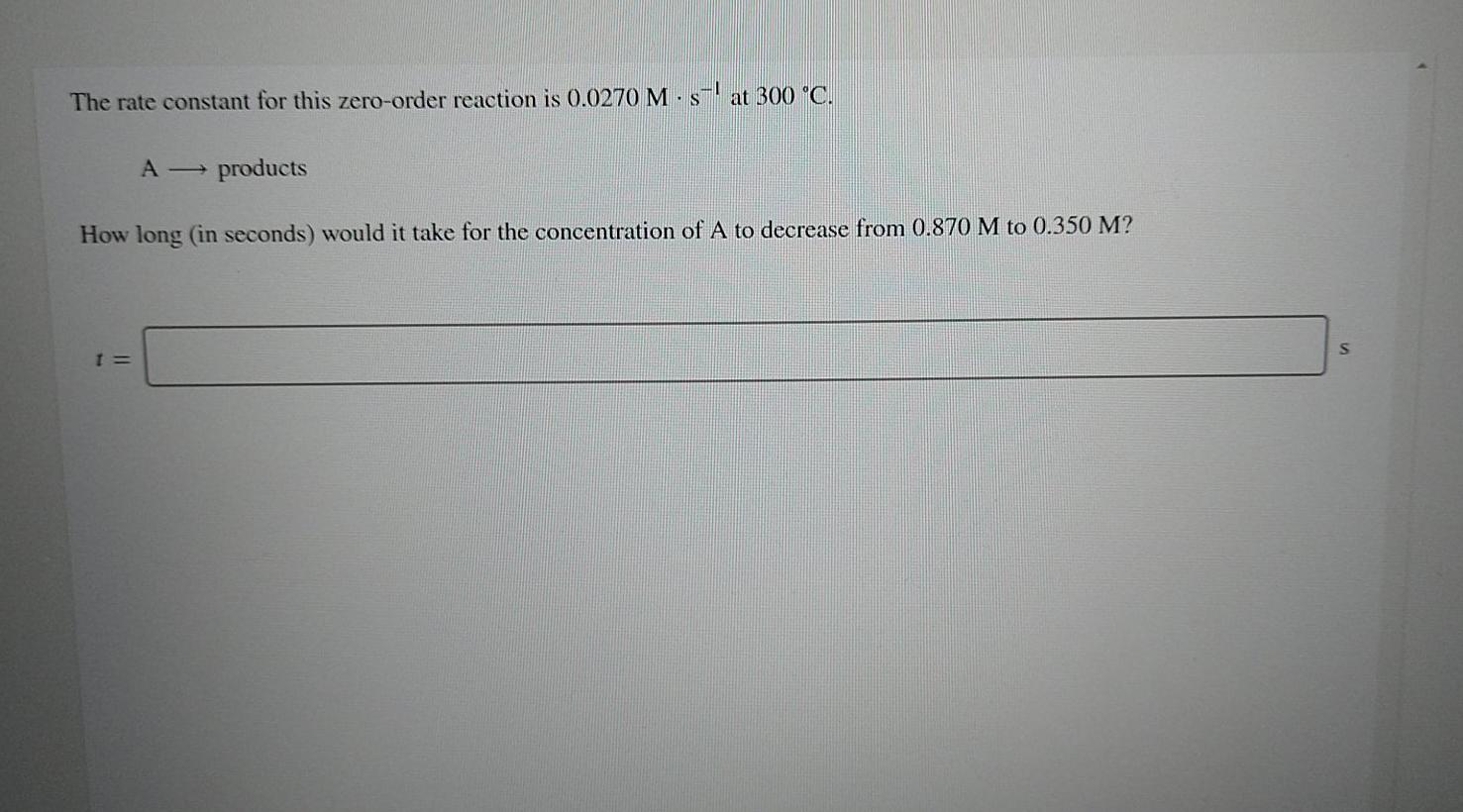
The rate constant for this zero-order reaction is 0.0270 M-s-1 at 300 C. A products How long (in seconds) would it take for the concentration of A to decrease from 0.870 M to 0.350 M? 1 =
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


