Question: The reaction 2 SO 2 ( g ) + O 2 ( g ) 2 SO 3 ( g ) is studied in the temperature
The reaction SOg OgSOg is studied in the temperature range K to K
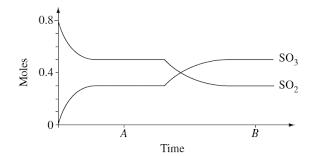
A number of moles were poured into a liter rigid container held along section A at a temperature of K
An equal amount of sulfur dioxide and oxygen, both in the gaseous state. The graph in front of you depicts the number of moles of the two sulfur oxides participating in the reaction as a function of time.
A Calculate the value of the equilibrium constant according to the concentrations at time point A Show calculations.
B Calculate the ratio between the pressure in the system at time point A and that at time T
third. Offer an explanation for the change that occurred in the system in the period of time that passed between point A and point B
D Calculate the concentration of the oxygen gas in the second equilibrium state, at point B
E Is the reaction to form SO from SO and oxygen endothermic or exothermic?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


