Question: The reaction 2NO(g) + O2(g) + 2NO2(g) is second order in NO and first order in Oz. When [NO] = 1.2 M and [02] =
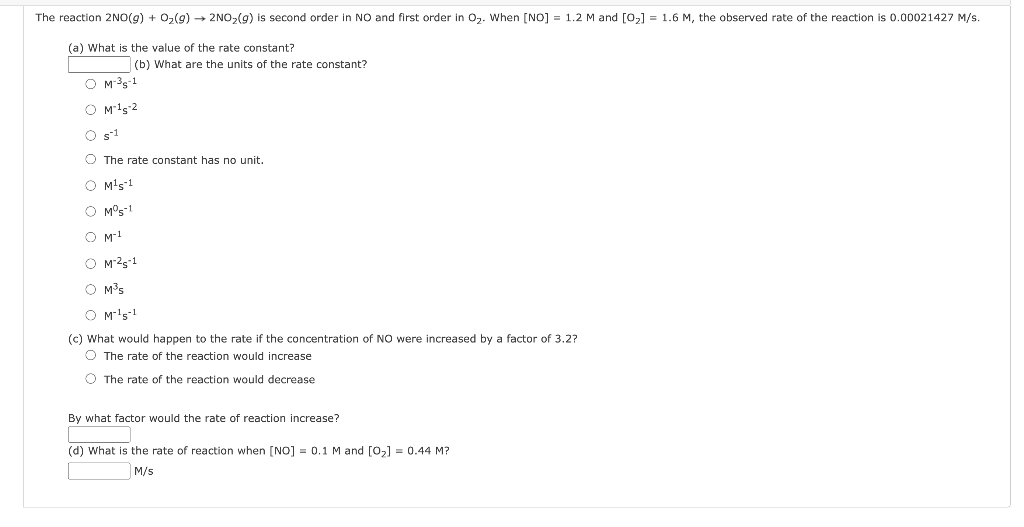
The reaction 2NO(g) + O2(g) + 2NO2(g) is second order in NO and first order in Oz. When [NO] = 1.2 M and [02] = 1.6 M, the observed rate of the reaction is 0.00021427 M/s. (a) What is the value of the rate constant? (b) What are the units of the rate constant? OM-35-1 OM 15-2 Os 1 The rate constant has no unit. O M's-1 0 M5-1 O M-1 OM-25-1 O Ms O M's-1 (c) What would happen to the rate if the concentration of NO were increased by a factor of 3.2? The rate of the reaction would increase O The rate of the reaction would decrease By what factor would the rate of reaction increase? (d) What is the rate of reaction when [NO] = 0.1 M and [02] = 0.44 M? M/S
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


