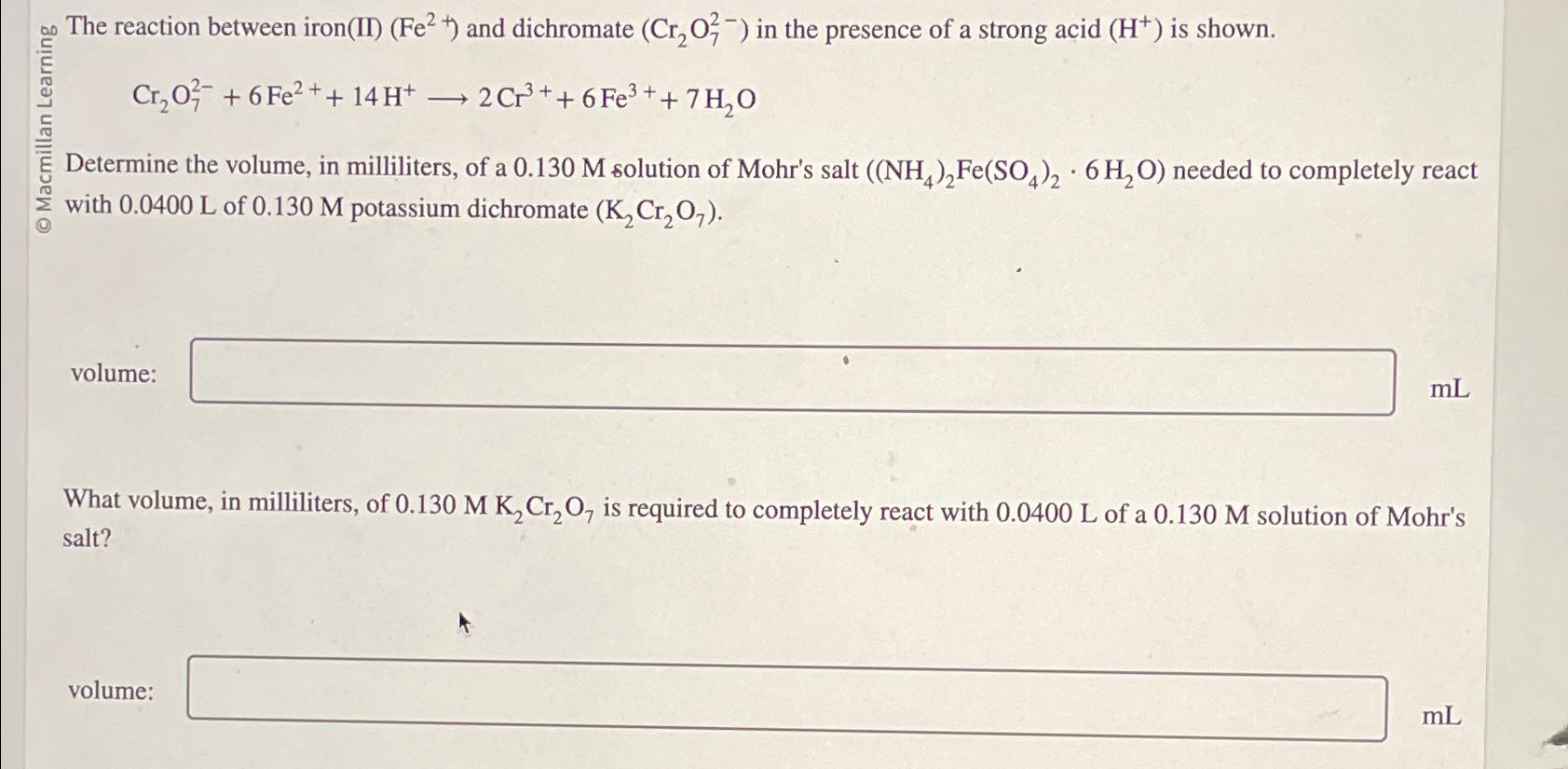
Question: The reaction between iron ( II ) ( F e 2 + ) and dichromate ( C r 2 O 7 2 - ) in
The reaction between ironII and dichromate in the presence of a strong acid is shown.
longrightarrow
Determine the volume, in milliliters, of a solution of Mohr's salt needed to completely react with of potassium dichromate
volume:
What volume, in milliliters, of is required to completely react with of a solution of Mohr's salt?
volume:
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


