Question: The reaction in solution between two compounds, A and B, is first order in B. The following results were obtained for the reaction at 25
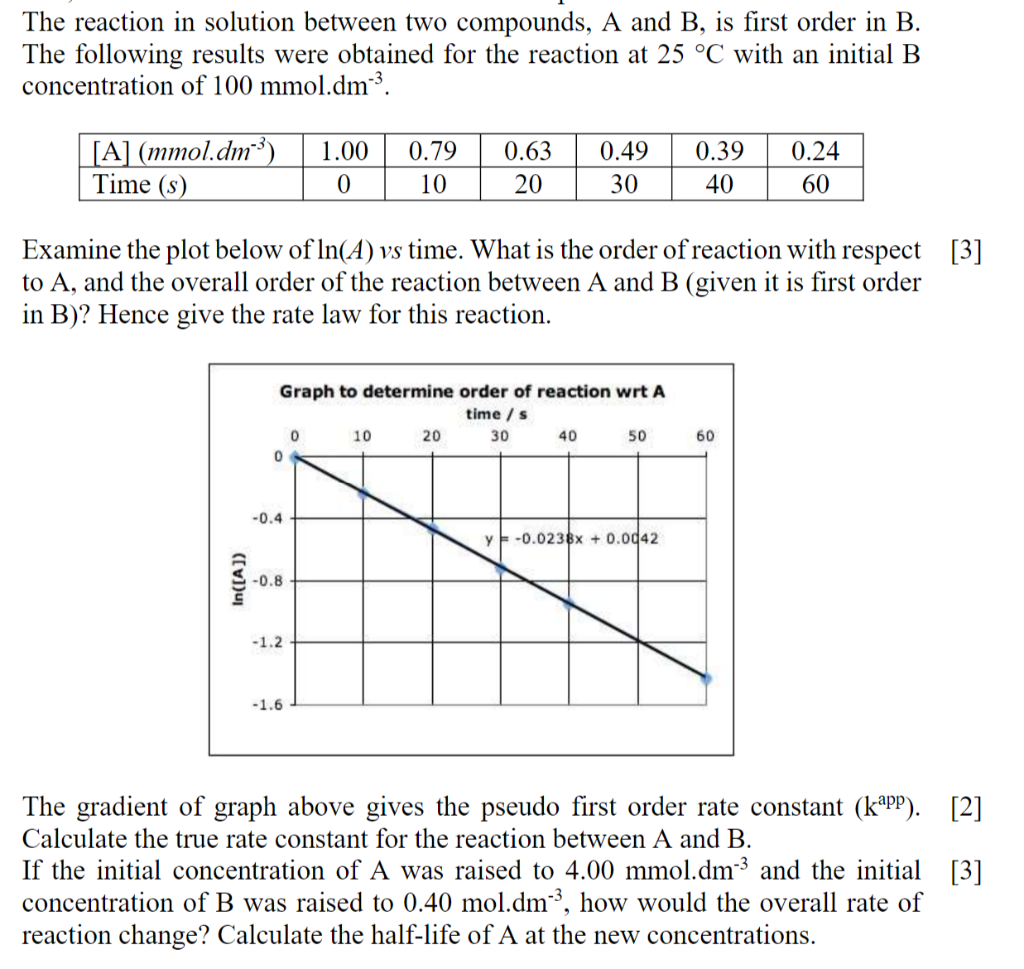
The reaction in solution between two compounds, A and B, is first order in B. The following results were obtained for the reaction at 25 C with an initial B concentration of 100 mmol.dm3. [A] (mmol.dm-3) 1.00 0 0.79 10 0.63 20 0.49 30 0.39 40 0.24 60 Time (s) Examine the plot below of In(A) vs time. What is the order of reaction with respect [3] to A, and the overall order of the reaction between A and B (given it is first order in B)? Hence give the rate law for this reaction. Graph to determine order of reaction wrt A time / s 0 10 20 30 40 50 0 60 -0.4 y -0.0236x + 0.0042 In([A]) -0.8 -1.2 -1.6 The gradient of graph above gives the pseudo first order rate constant (kapp). [2] Calculate the true rate constant for the reaction between A and B. If the initial concentration of A was raised to 4.00 mmol.dm" and the initial [3] concentration of B was raised to 0.40 mol.dm-3, how would the overall rate of reaction change? Calculate the half-life of A at the new concentrations
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


