Question: The reaction N 2 O 4 ( g ) 2 N O 2 ( g ) occurs in an evacuated container at 3 7 3
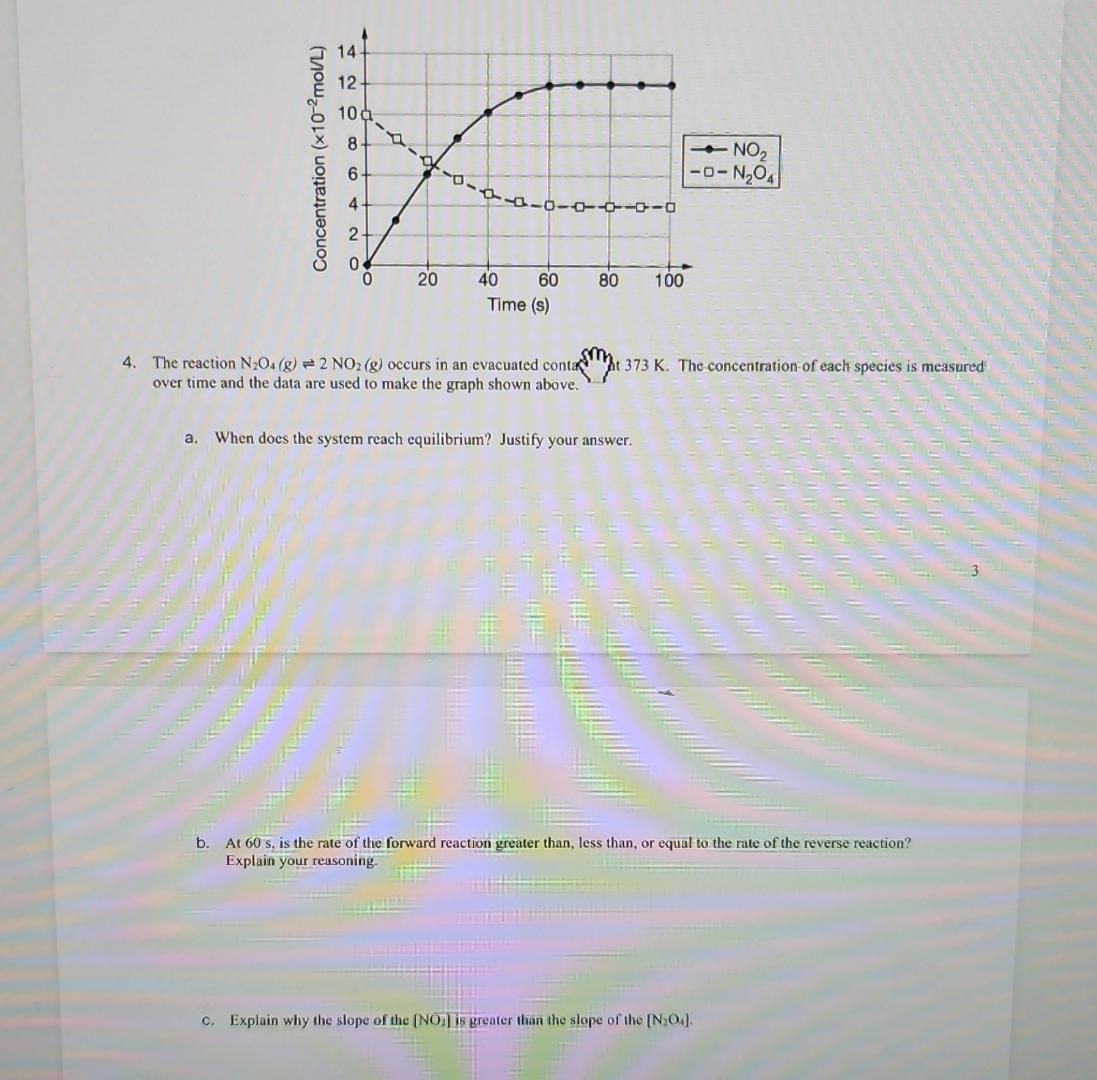
The reaction occurs in an evacuated container at The concentration of each species is measured
over time and the data are used to make the graph shown above.
a When does the system reach equilibrium? Justify your answer.
b At is the rate of the forward reaction greater than, less than, or equal to the rate of the reverse reaction?
Explain your reasoning.
C Explain why the slope of the is greater than the slope of the
The reaction occurs in an evacuated contash The concentrationof each species is measured over time and the data are used to make the graph shown above.
a When does the system reach equilibrium? Justify your answer.
b At is the rate of the forward reaction greater than, less than, or equal to the rate of the reverse reaction?
Explain your reasoning.
c Explain why the slope of the is greater than the slope of the
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


