Question: the reaction - OCT (a) e or (aq) +CT (aq) lowing mechanism has been proposed: (20 pts) OCT (ap)+H.00. > HOCI + OH (a (o)-
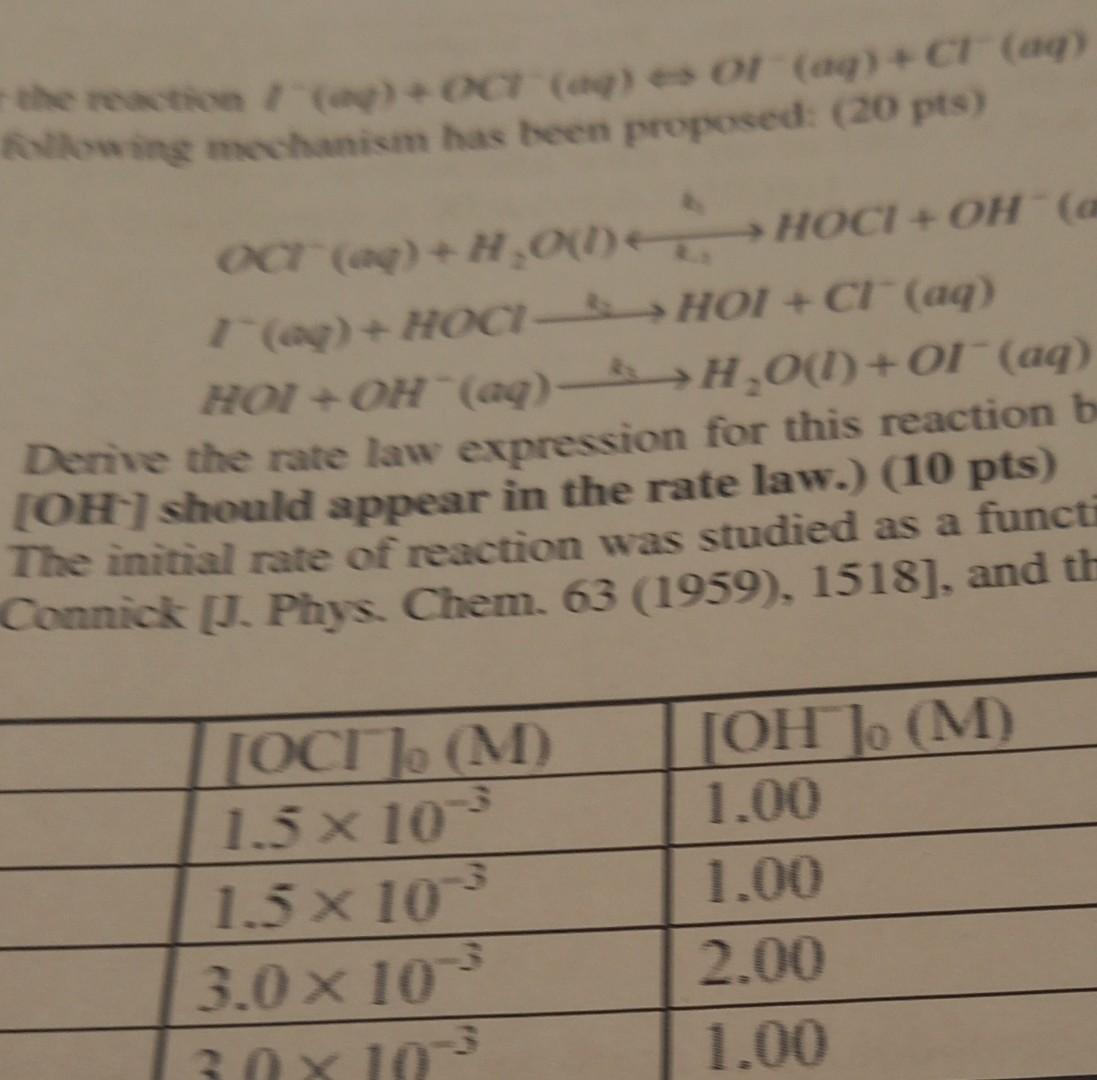
the reaction - OCT (a) e or (aq) +CT (aq) lowing mechanism has been proposed: (20 pts) OCT (ap)+H.00. > HOCI + OH (a (o)- HOCHOI +C (aq) HOI + OH(aq) H,001)+1(aq) Derive the rate law expression for this reaction b (OH) should appear in the rate law.) (10 pts) The initial rate of reaction was studied as a functi Connick [J. Phys. Chem. 63 (1959), 1518], and th [OC] (M) 1.5 x 10 1.5 x 10 3.0 x 10 [ [OH](M) 1.00 1.00 2.00 1.00 30X10 the reaction - OCT (a) e or (aq) +CT (aq) lowing mechanism has been proposed: (20 pts) OCT (ap)+H.00. > HOCI + OH (a (o)- HOCHOI +C (aq) HOI + OH(aq) H,001)+1(aq) Derive the rate law expression for this reaction b (OH) should appear in the rate law.) (10 pts) The initial rate of reaction was studied as a functi Connick [J. Phys. Chem. 63 (1959), 1518], and th [OC] (M) 1.5 x 10 1.5 x 10 3.0 x 10 [ [OH](M) 1.00 1.00 2.00 1.00 30X10
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


