Question: The reactuon A--> B + C is carried out at a particular temperature. The observed date is recorded at night. Graph the data to determine
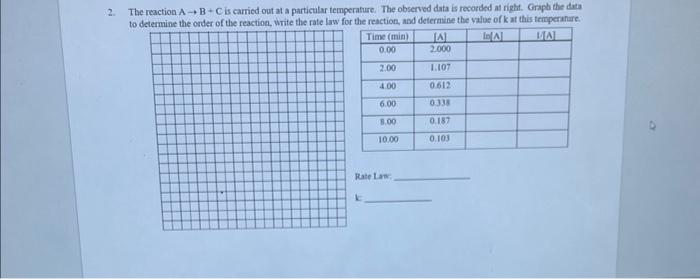
The reaction AB+C is carried out at a particular temperature. The observed data is recorded at righ. Graph the data to determine the order of the reaction. Write the rate law for the reaction. and determine the value of k at this temperanure. Rate Lan: 1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


