Question: The relative volatility ( 1 2 ) for the system consisting of carbon sulphide ( 1 ) and acetone ( 2 ) at 3 5
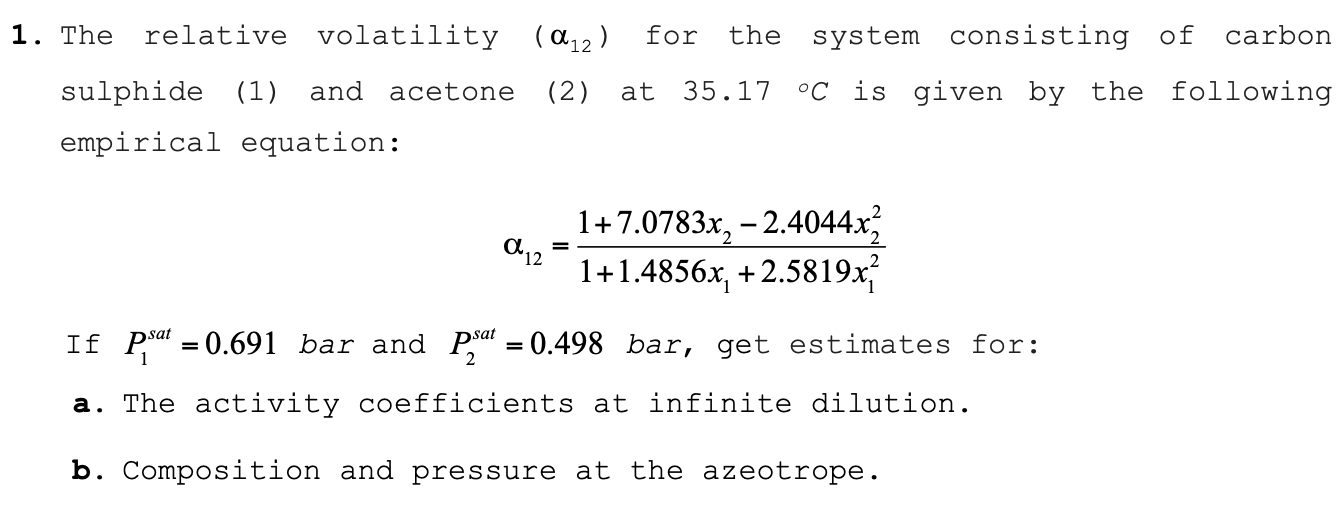
The relative volatility for the system consisting of carbon
sulphide and acetone at is given by the following
empirical equation:
If bar and bar, get estimates for:
a The activity coefficients at infinite dilution.
b Composition and pressure at the azeotrope.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


