Question: The series reactions 2 A B ? ( 2 ) l o n g r i g h t a r r o w 4
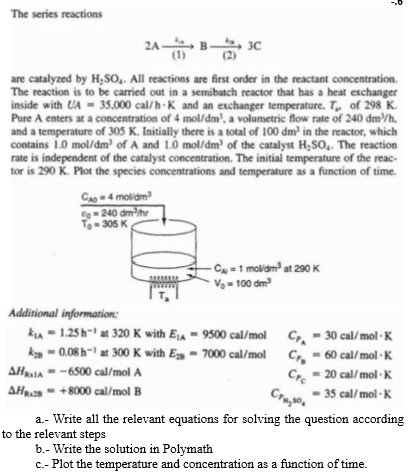
The series reactions
are catalyzed by All reactions are first order in the reactant concentration.
The reaction is to be carried out in a semibatch reactor that has a heat exchanger
inside with and an exchanger temperature, of
Pure A enters at a concentration of a volumetric flow rate of
and a temperature of Initially there is a total of in the reactor, which
contains of A and of the catalyst The reaction
rate is independent of the catalyst concentration. The initial temperature of the reac
tor is Plot the species concentrations and temperature as a function of time.
Additional information:
with
with
olA
olB
a Write all the relevant equations for solving the question according
to the relevant steps
b Write the solution in Polymath
c Plot the temperature and concentration as a function of time.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


