Question: THE SPECIAL PERIODIC TABLE IS ON HERE. PLEASE HELP WITH ALL THANK YOU Exercise 2 a) Calculate the mass percent of a solution containing 13.0
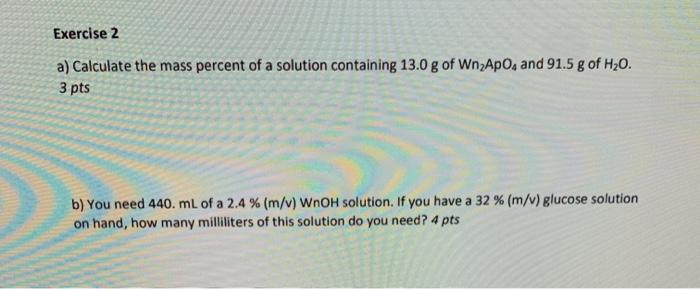

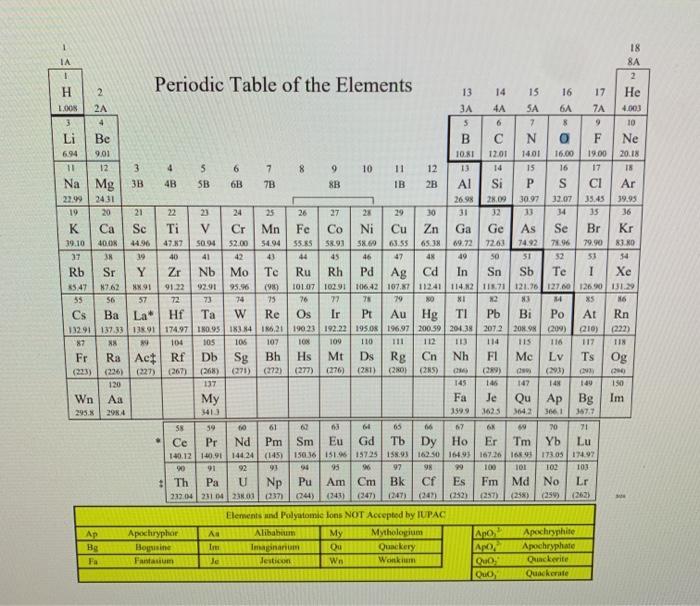
Exercise 2 a) Calculate the mass percent of a solution containing 13.0 g of WnApo, and 91.5 g of H2O. 3 pts b) You need 440. mL of a 2.4 % (m/v) WnOH solution. If you have a 32 % (m/v) glucose solution on hand, how many milliliters of this solution do you need? 4 pts Exercise 3 a) Water is a polar solvent and hexane (C6H14) is a nonpolar solvent. In which solvent is Wn Quoz (ionic compound) more likely to be soluble? 2 pts b) Indicate the compartment that will increase in volume for the following pairs of solutions separated by a semipermeable membrane: 2 M sucrose or 3 M sucrose? 2 pts c) Calculate the mass of solute needed to prepare 1300 mL of a 7.0 % (m/v) Wn2ApOx solution. 3 pts END Exercise 4 What is the molarity of a solution containing 251 g of WnCl in 147 mL of solution? Use special periodic table. 7 pts END Exercise 5 Calculate the grams of solute needed to prepare 6.40 L of a 0.120 M Aa(OH)solution. Use the special periodic table. 7 pts Periodic Table of the Elements 6A Ge 18 IA 8A 1 2 H 2 13 15 16 17 He 1.008 2A 4A SA 7A 4.00 3 4 5 6 7 8 9 10 Li Be B N 0 F Ne 6.94 9.01 10.81 12.01 14.01 16.00 19.00 20.18 12 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Na Mg 3B 4B SB 6B 7B SB IB 2B Al Si P S C1 Ar 22.99 2431 26.98 28.09 30.97 12.07 35.45 39.95 19 20 21 22 23 25 27 28 29 30 31 13 34 35 36 K Ca Sc Ti V Cr Mn Fe Ni Cu Zn Ga As Se Br Kr 39.10 40.0 44.96 47.17 50.94 $2.00 SE04 55.RS 58.93 SX 60 63.55 65. IN 69.72 72637492 74.96 79.90 81.NO 37 38 19 40 41 42 43 44 45 46 47 48 49 50 51 32 53 54 Rb Sr Y Zr Nb Mo Te Ru Rh Pd Ag cd In Sn Sb Te I Xe 3547 1762 NX 91 91.22 92.91 95.9 (98) 101.07 1029 106.42 107.711241 114.82 115.71 121.76127.00 126,90 131.29 35 56 57 72 73 74 75 76 77 78 709 SO NI 2 3 X5 6 Cs Ba La* f Ta W Os Ir Pt Au Hg TI Pb Bi Po At Rn 13291 137.33 138 91 174.97 180.95 183.84 186,21 1923 192.22 195.0% 196.97 200.5920438 2072 2019 (209) 210) (222) 87 99 104 105 107 10X 109 110 111 112 114 115 116 117 118 Fr Ra Act Rf Db Sg Bh Hs Mt Ds Rg Cn Nh F1 Mc Lv Ts (223) (226) (227) (267) (268) (271) (272) (277) (276) (281) (203 (285) (289) N 20 120 137 145 146 147 14 149 150 Wn Aa My Fa Je Qu Ap Bg | Im 2984 341 1599 3625 1642 3661 367.7 56 59 00 61 6 67 63 9 70 71 Ce Pr Nd Pm Sm Eu Gd T | Dy Ho Er Tm Yb Lu 140.12 140.91 144.24 (145) 15036151915725158.93 162.50 164.93 167.2616173.05 17497 90 91 92 93 94 95 97 98 99 100 101 102 103 # Th Pa U Np Pu Am Cm Bk cf Es Fm Md No Lr 232,04 231 042303 (237) (244) (1247) (24) (252) (258) (259) Re 105 Og . Bg Fa ARO Apocryphor Bogumine Fantasium Elements and Polyatomie lons NOT Accepted by IUPAC Alibabu My Mythologium Ou Quackery Jestico wo Wanium As Im Je Apochryphine Apochryphate Quickerite Quackerate Quo Quo
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


