Question: The steady-state washing process shown in Figure 1 was set up to take the Raw Pigment and remove as much of the undesired salt from
The steady-state washing process shown in Figure 1 was set up to take the Raw Pigment and remove as much of the undesired salt from the desired TiO2 as possible, so that the salt can be disposed of in the Waste Water stream, leaving the valuable TiO2 in the Washed Products stream. If the process is running with a yield of 92% TiO2 and a selectivity of 7 in Stream B, then calculate the overall rate of the Wash Water stream. For this question, include: Given, Find, Assumptions, Problem types/features, Calculations, and Conclusions. Also include full balances using the General Balance Equation, where necessary.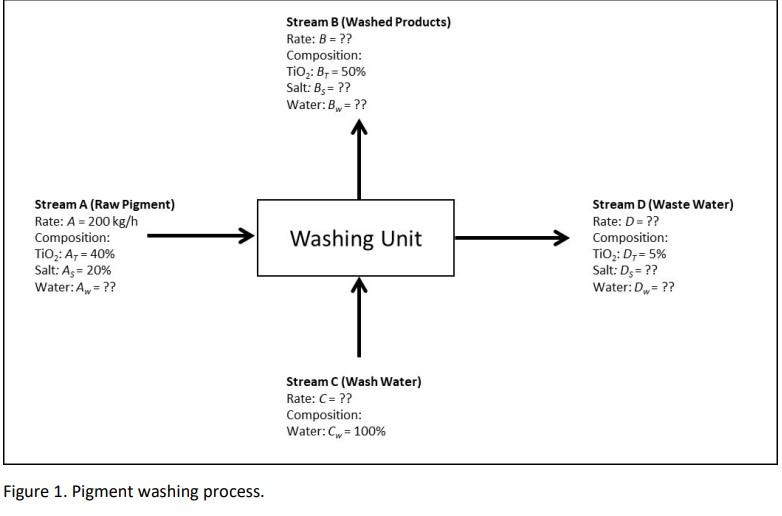
Stream B (Washed Products) Rate: B = ?? Composition: TiO : Br = 50% Salt: Bs= ?? Water: B = ?? Stream A (Raw Pigment) Rate: A = 200 kg/h Composition: TiO2: A = 40% Salt: A = 20% Water:Aw= ?? Washing Unit Stream D(Waste Water) Rate: D = ?? Composition: TiO2: D,- 5% Salt: Ds = ?? Water: Dw=?? Stream (Wash Water) Rate: C= ?? Composition: Water:Cw = 100% Figure 1. Pigment washing process
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


