Question: The student id is 4,6,8 for example 1. An engineer is designing an absorption system in order to remove sulfur dioxide (SO2) from wet flue
The student id is 4,6,8 for example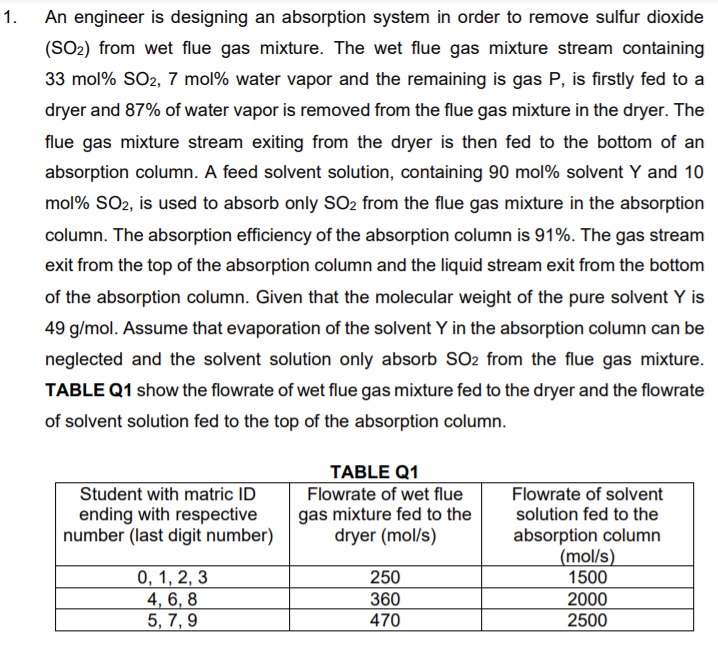

1. An engineer is designing an absorption system in order to remove sulfur dioxide (SO2) from wet flue gas mixture. The wet flue gas mixture stream containing 33 mol% SO2, 7 mol% water vapor and the remaining is gas P, is firstly fed to a dryer and 87% of water vapor is removed from the flue gas mixture in the dryer. The flue gas mixture stream exiting from the dryer is then fed to the bottom of an absorption column. A feed solvent solution, containing 90 mol% solvent Y and 10 mol% SO2, is used to absorb only SO2 from the flue gas mixture in the absorption column. The absorption efficiency of the absorption column is 91%. The gas stream exit from the top of the absorption column and the liquid stream exit from the bottom of the absorption column. Given that the molecular weight of the pure solvent Y is 49 g/mol. Assume that evaporation of the solvent Y in the absorption column can be neglected and the solvent solution only absorb SO2 from the flue gas mixture. TABLE Q1 show the flowrate of wet flue gas mixture fed to the dryer and the flowrate of solvent solution fed to the top of the absorption column. Student with matric ID ending with respective number (last digit number) TABLE Q1 Flowrate of wet flue gas mixture fed to the dryer (mol/s) Flowrate of solvent solution fed to the absorption column (mol/s) 1500 2000 2500 0, 1, 2, 3 4, 6, 8 5, 7, 9 250 360 470 b. Determine the molar composition for all components in the exit gas stream and the exit liquid stream, respectively. [20 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


