Question: The symbol shown represents the isotopic notation for the isotope of an element: 1) Given the element Chromium (Cr) what is the value of Z
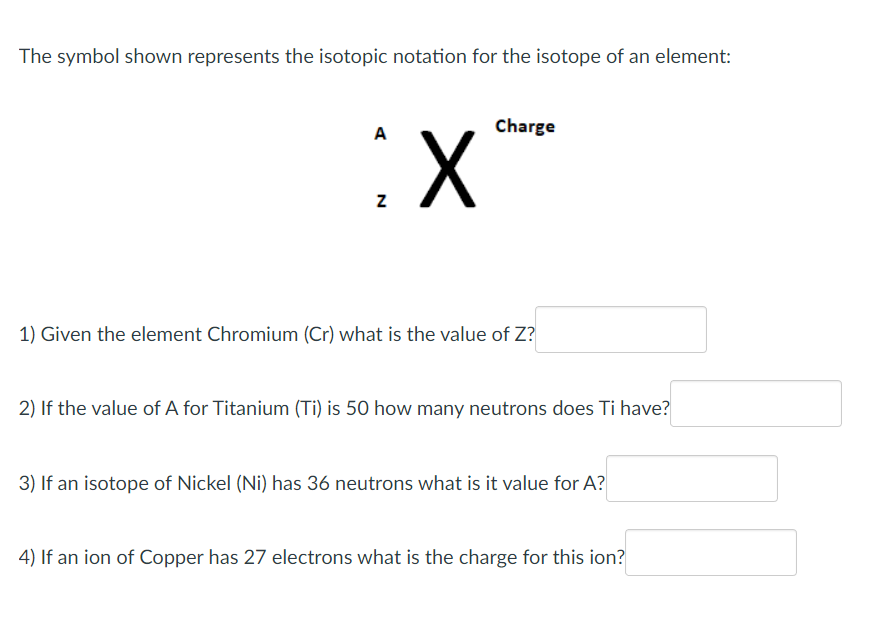
The symbol shown represents the isotopic notation for the isotope of an element: 1) Given the element Chromium (Cr) what is the value of Z : 2) If the value of A for Titanium (Ti) is 50 how many neutrons does Ti have? 3) If an isotope of Nickel (Ni) has 36 neutrons what is it value for A ? 4) If an ion of Copper has 27 electrons what is the charge for this ion
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


