Question: The table below is from a study investigating the feasibility of using aerobic bacteria to mediate oxidation of various chlorinated hydrocarbons. The system under study
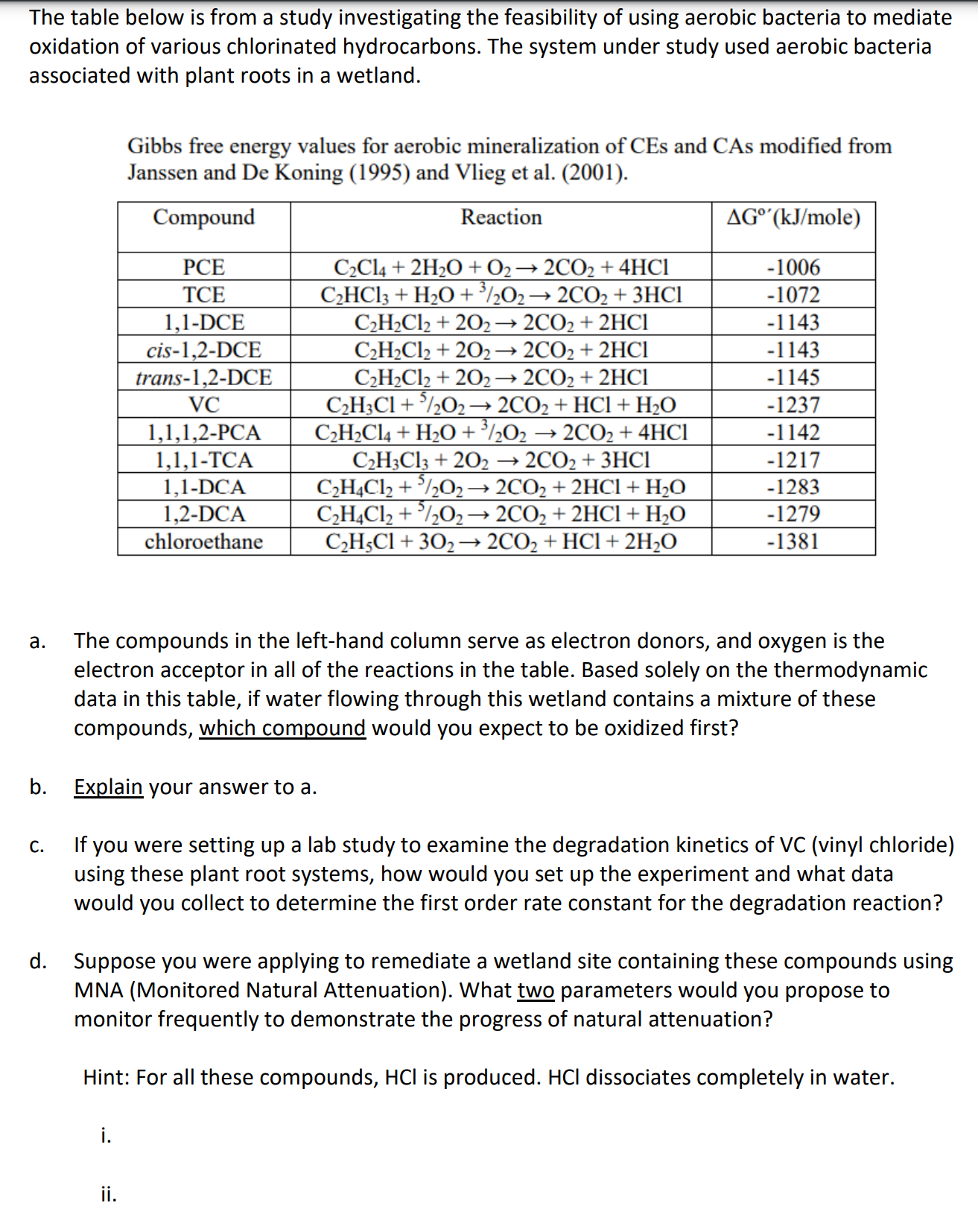
The table below is from a study investigating the feasibility of using aerobic bacteria to mediate oxidation of various chlorinated hydrocarbons. The system under study used aerobic bacteria associated with plant roots in a wetland. Gibbs free energy values for aerobic mineralization of CEs and CAs modified from Janssen and De Koning (1995) and Vlieg et al. (2001). a. The compounds in the left-hand column serve as electron donors, and oxygen is the electron acceptor in all of the reactions in the table. Based solely on the thermodynamic data in this table, if water flowing through this wetland contains a mixture of these compounds, which compound would you expect to be oxidized first? b. Explain your answer to a. c. If you were setting up a lab study to examine the degradation kinetics of VC (vinyl chloride) using these plant root systems, how would you set up the experiment and what data would you collect to determine the first order rate constant for the degradation reaction? d. Suppose you were applying to remediate a wetland site containing these compounds using MNA (Monitored Natural Attenuation). What two parameters would you propose to monitor frequently to demonstrate the progress of natural attenuation? Hint: For all these compounds, HCl is produced. HCl dissociates completely in water
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


