Question: the table below is the data needed for this question QUESTION 5 Calculate the amount of the liquid left in the still at the end
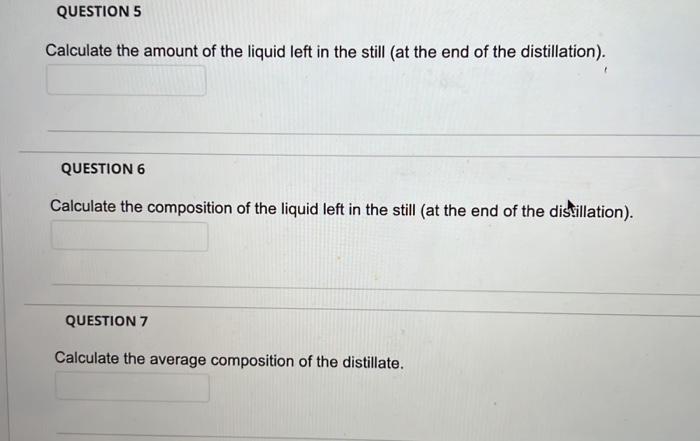
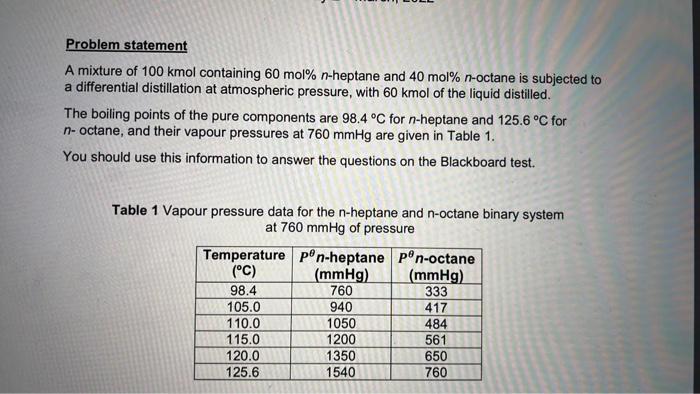
QUESTION 5 Calculate the amount of the liquid left in the still at the end of the distillation). QUESTION 6 Calculate the composition of the liquid left in the still (at the end of the distillation). QUESTION 7 Calculate the average composition of the distillate. Problem statement A mixture of 100 kmol containing 60 mol% n-heptane and 40 mol% n-octane is subjected to a differential distillation at atmospheric pressure, with 60 kmol of the liquid distilled. The boiling points of the pure components are 98.4 C for n-heptane and 125.6 C for n-octane, and their vapour pressures at 760 mmHg are given in Table 1. You should use this information to answer the questions on the Blackboard test. Table 1 Vapour pressure data for the n-heptane and n-octane binary system at 760 mmHg of pressure Temperature pn-heptane pon-octane (C) (mmHg) (mmHg) 98.4 760 333 105.0 940 417 110.0 1050 484 115.0 1200 561 120.0 1350 650 125.6 1540 760
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


