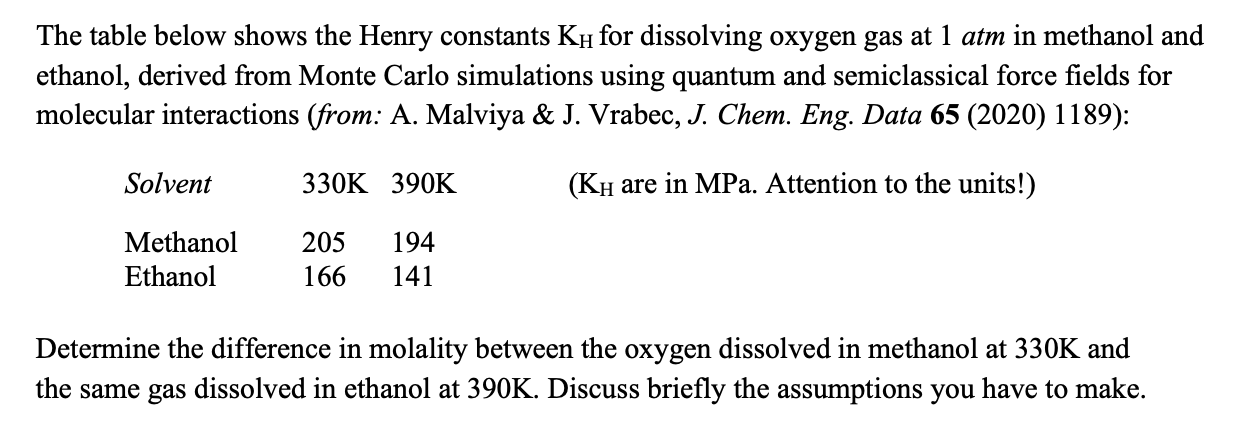
Question: The table below shows the Henry constants K H for dissolving oxygen gas at 1 atm in methanol and ethanol, derived from Monte Carlo simulations
The table below shows the Henry constants for dissolving oxygen gas at atm in methanol and
ethanol, derived from Monte Carlo simulations using quantum and semiclassical force fields for
molecular interactions from: A Malviya & J Vrabec, J Chem. Eng. Data :
Determine the difference in molality between the oxygen dissolved in methanol at and
the same gas dissolved in ethanol at Discuss briefly the assumptions you have to make.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


