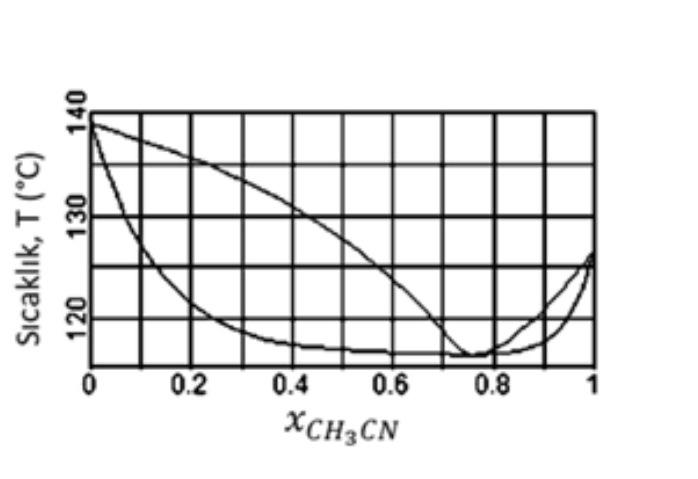
Question: The temperature component graph formed by the binary mixture of Water - Acetonitrile under 3,5 bar pressure is as on the side. By using this
The temperature component graph formed by the binary mixture of Water - Acetonitrile under 3,5 bar pressure is as on the side. By using this chart; Temperature, T ("C)
A-) When the concentration of acetonitrile (CH,CN) mixed with water is analyzed, it is observed that acetonitrile has 0,43 mole fraction in this mixture. What is the approximate mole fraction of acetonitrile in the distillate (in the deposited distillate) when distilled by simple (ordinary) distillation?
B-) If 100ml of water and 100ml of acetonitrile are mixed under the same conditions, would the volume of the mixture be 200ml, more or less?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


