Question: The top layer looks as shown below. Mark the atoms that form a face of the unit cell cube by annotating the image below. Question


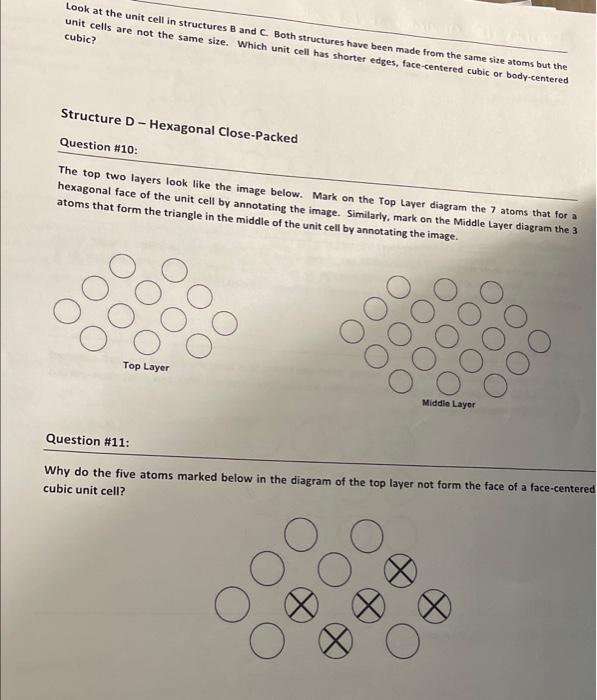

The top layer looks as shown below. Mark the atoms that form a face of the unit cell cube by annotating the image below. Question #2: O In which layer is the opposite face to the one marked in Question #1, the bottom or the middle? Question #3: How many marbles are involved in the making up of the unit cell? (Count the marbles and don't divide them between cells) Structure B Question #4: The top layer looks as shown below. Mark the atoms that form a face of the unit cell cube by annotating the image below. Look carefully and remember that all edges of a cube have the same length. The front sloping edge looks like the image below. Mark the atoms that form a hexagon of the cubic close- packed structure (a total of 7 atoms). Question #6: Compare structures A and B. To help you with this, remove 7 marbles from structure A as follows: from the top layer remove one marble from the right front corner. From the middle layer remove the two marbles now showing in the right front corner. From the bottom layer remove the four marbles now showing in the right front corner. Look at the sloping face that you have uncovered and explain what you see. 000 OOO Structure C Question #7: The top layer looks as shown below. Mark the atoms that form a face of the unit cell cube by annotating the image below. DOO Question #8: How many marbles are involved in the making up of the unit cell? Look at the unit cell in structures B and C Both structures have been made from the same size atoms but the unit cells are not the same size. Which unit cell has shorter edges, face-centered cubic or body-centered cubic? Structure D-Hexagonal Close-Packed Question #10: The top two layers look like the image below. Mark on the Top Layer diagram the 7 atoms that for a hexagonal face of the unit cell by annotating the image. Similarly, mark on the Middle Layer diagram the 3 atoms that form the triangle in the middle of the unit cell by annotating the image. oo Top Layer OOC Dool ool Middle Layer Question #11: Why do the five atoms marked below in the diagram of the top layer not form the face of a face-centered cubic unit cell? O Ol Question #12: The top two layers look like the image below. Mark on the top layer diagram the 7 atoms that forma hexagonal face of the unit cell. Mark on the Middle Layer diagram the 3 atoms that form the triangle in the middle of the unit cell. Top layer od O Question #13: Miodic layer Mark on the top layer diagram of Question #12 the places where the 3 atoms that form the triangle in the fourth (and first) layer are. You have enough marbles to add these 3 atoms to your structure but do i carefully since the weight of the marbles may be enough to collapse the structure. Question #14: The front sloping face looks like the image below. Mark the atoms that form the face of the tace-centered cubic unit cell by annotating the image. This is possible because face-centered cubic and cubic close-packed are two names for the same structure. OOOO Question #15: Compare this structure with structure B
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


