Question: The trend in second ionization energy for the elements from lithium to fluorine is not a regular one. Predict which of these elements has
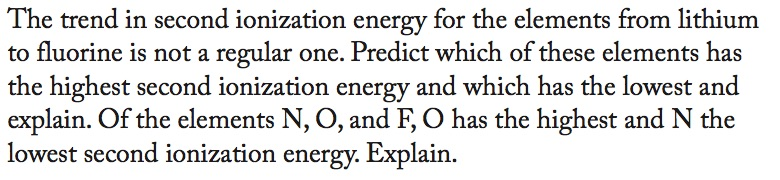
The trend in second ionization energy for the elements from lithium to fluorine is not a regular one. Predict which of these elements has the highest second ionization energy and which has the lowest and explain. Of the elements N, O, and F, O has the highest and N the lowest second ionization energy. Explain.
Step by Step Solution
3.41 Rating (145 Votes )
There are 3 Steps involved in it
Lit 152 B 15 Bet 152 25 15 252 15 282 24 152 282 26 3 to ta TTT I52 252 28 2p f ... View full answer

Get step-by-step solutions from verified subject matter experts


