Question: The two organic compounds shown below are isomers- different compounds - not resonance forms of the same compound. (I moved atoms in the skeleton.) One
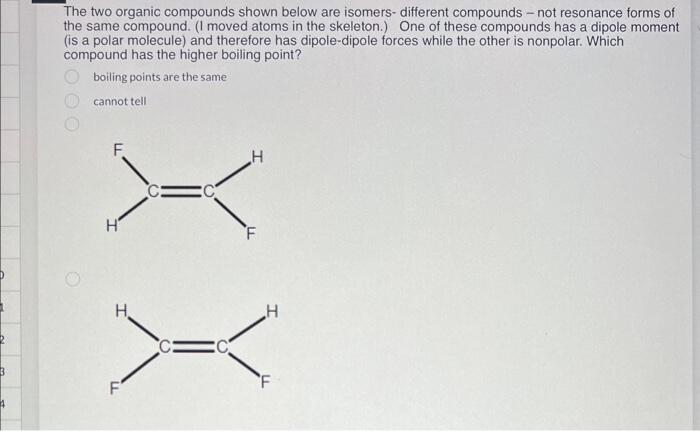
The two organic compounds shown below are isomers- different compounds - not resonance forms of the same compound. (I moved atoms in the skeleton.) One of these compounds has a dipole moment (is a polar molecule) and therefore has dipole-dipole forces while the other is nonpolar. Which compound has the higher boiling point? boiling points are the same cannot tell
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


