Question: The valuable compound diborane, B2H6, can be made by the reaction 2 NaBH4 (s) + 12 (s) B2H6 (g) + 2 Nal(s) + H2
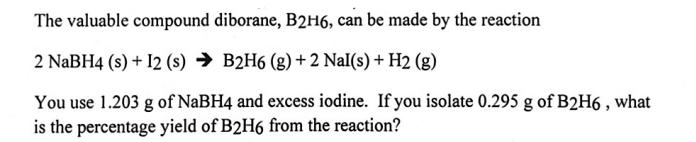
The valuable compound diborane, B2H6, can be made by the reaction 2 NaBH4 (s) + 12 (s) B2H6 (g) + 2 Nal(s) + H2 (g) You use 1.203 g of NaBH4 and excess iodine. If you isolate 0.295 g of B2H6, what is the percentage yield of B2H6 from the reaction?
Step by Step Solution
★★★★★
3.50 Rating (150 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

The detailed ... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


