Question: The water-gas shift reaction process described in example 9.5-3 is now carried out in a plant operating on small (distributed) scale. The feed contains 53
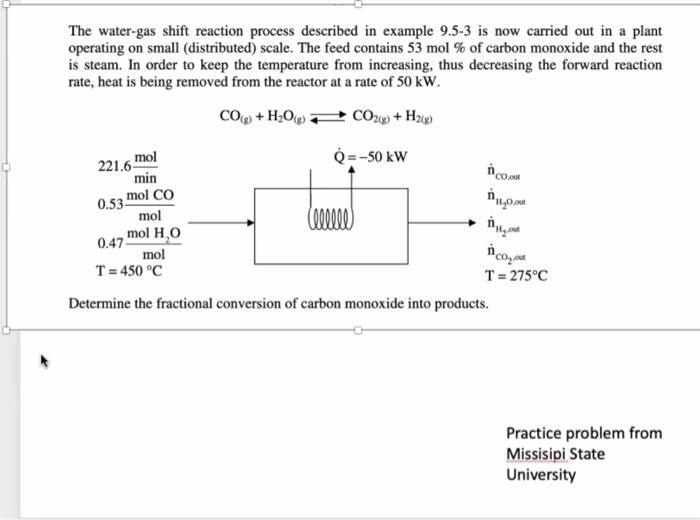
The water-gas shift reaction process described in example 9.53 is now carried out in a plant operating on small (distributed) scale. The feed contains 53mol% of carbon monoxide and the rest is steam. In order to keep the temperature from increasing, thus decreasing the forward reaction rate, heat is being removed from the reactor at a rate of 50kW. CO(g)+H2O(g)CO2(g)+H2(g) Determine the fractional conversion of carbon monoxide into products
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


