Question: Theory and Practice It is common to apply a procedure to a system with known properties to demonstrate that accurate data is being obtained when
Theory and Practice
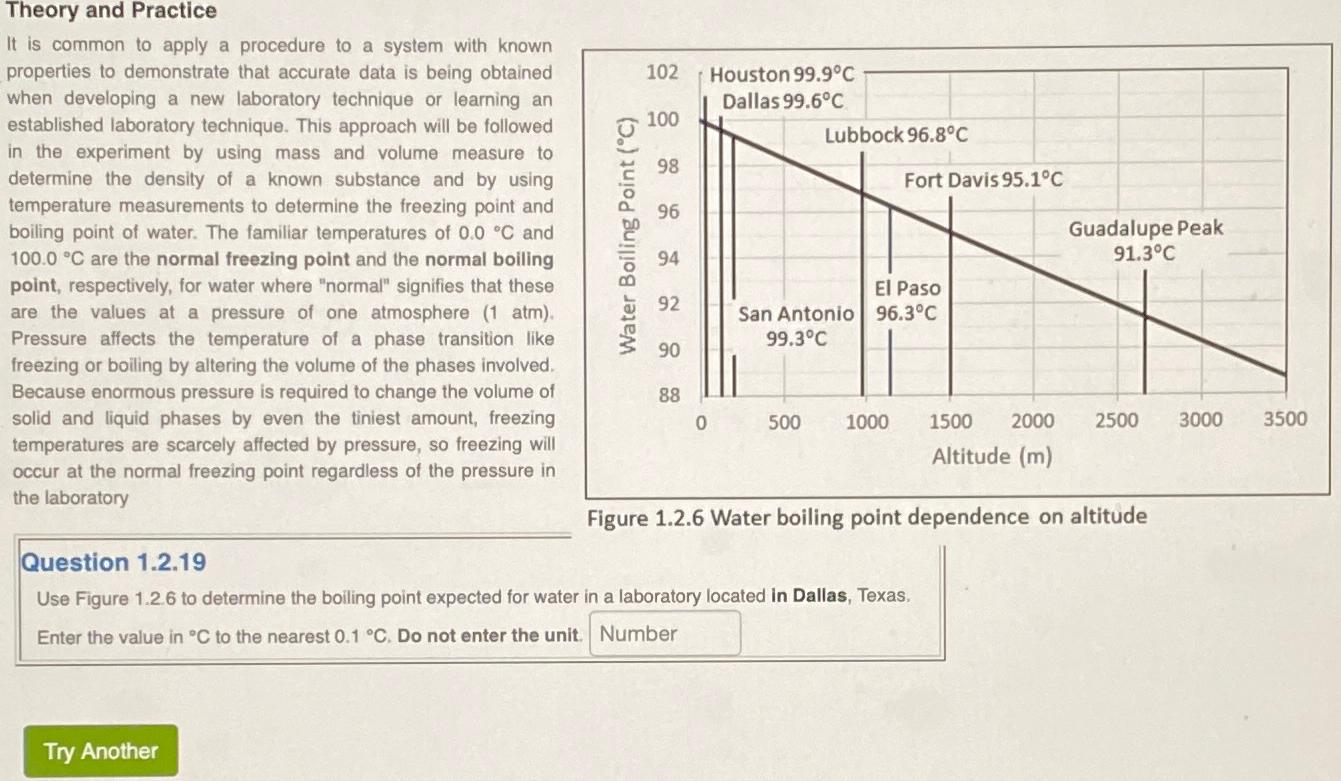
It is common to apply a procedure to a system with known properties to demonstrate that accurate data is being obtained when developing a new laboratory technique or learning an established laboratory technique. This approach will be followed in the experiment by using mass and volume measure to determine the density of a known substance and by using temperature measurements to determine the freezing point and boiling point of water. The familiar temperatures of and are the normal freezing point and the normal boiling point, respectively, for water where "normal" signifies that these are the values at a pressure of one atmosphere atm Pressure affects the temperature of a phase transition like freezing or boiling by altering the volume of the phases involved. Because enormous pressure is required to change the volume of solid and liquid phases by even the tiniest amount, freezing temperatures are scarcely affected by pressure, so freezing will occur at the normal freezing point regardless of the pressure in the laboratory
Question
Use Figure to determine the boiling point expected for water in a laboratory located in Dallas, Texas.
Enter the value in to the nearest Do not enter the unit.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


