Question: There are important relations between atom and bulk material (Figure 2). (a) Explain the simplified concept of an atom. (b) Explain the significance of the
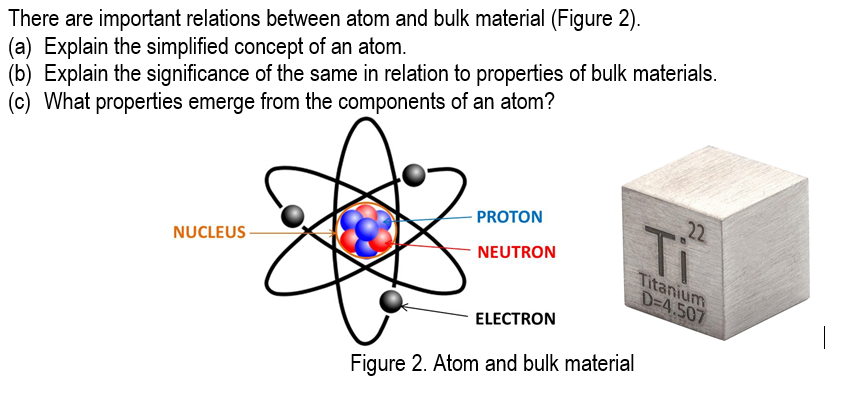
There are important relations between atom and bulk material (Figure 2). (a) Explain the simplified concept of an atom. (b) Explain the significance of the same in relation to properties of bulk materials. (C) What properties emerge from the components of an atom? PROTON NUCLEUS 22 NEUTRON T Titanium D=4.507 ELECTRON Figure 2. Atom and bulk material
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


