Question: There are parts (A,B,C,D) to this question. Each answer should be in the form of a ratio. A series of reactions carried out in separate
There are parts (A,B,C,D) to this question. Each answer should be in the form of a ratio. 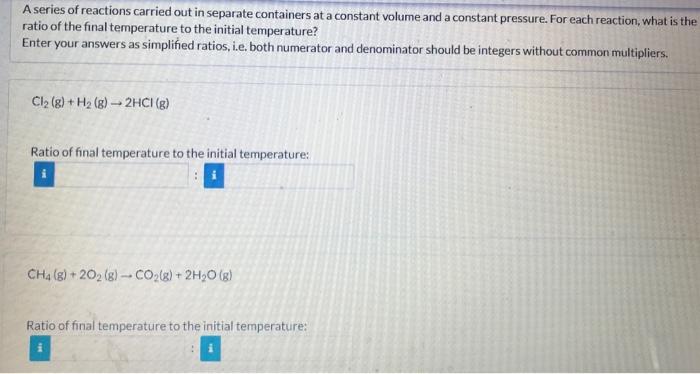
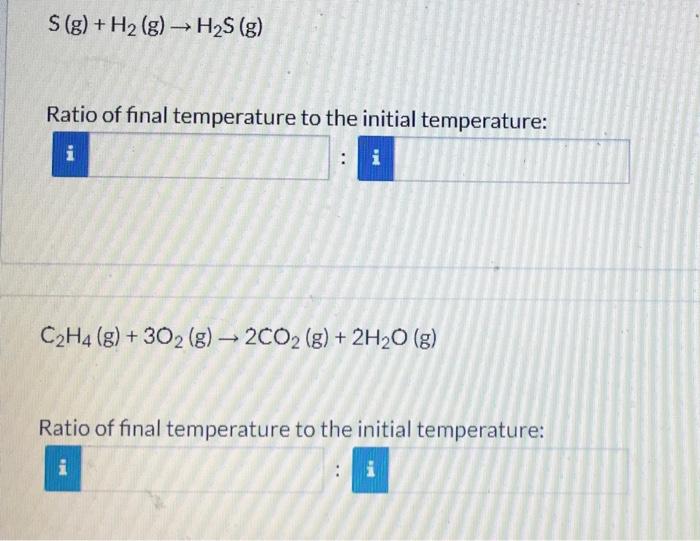


A series of reactions carried out in separate containers at a constant volume and a constant pressure. For each reaction, what is the ratio of the final temperature to the initial temperature? Enter your answers as simplified ratios, i.e. both numerator and denominator should be integers without common multipliers. Cl2(g)+H2(g)2HCl(g) Ratio of final temperature to the initial temperature: CH4(g)+2O2(g)CO2(g)+2H2O(g) Ratio of final temperature to the initial temperature: i S(g)+H2(g)H2S(g) Ratio of final temperature to the initial temperature: C2H4(g)+3O2(g)2CO2(g)+2H2O(g) Ratio of final temperature to the initial temperature
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


