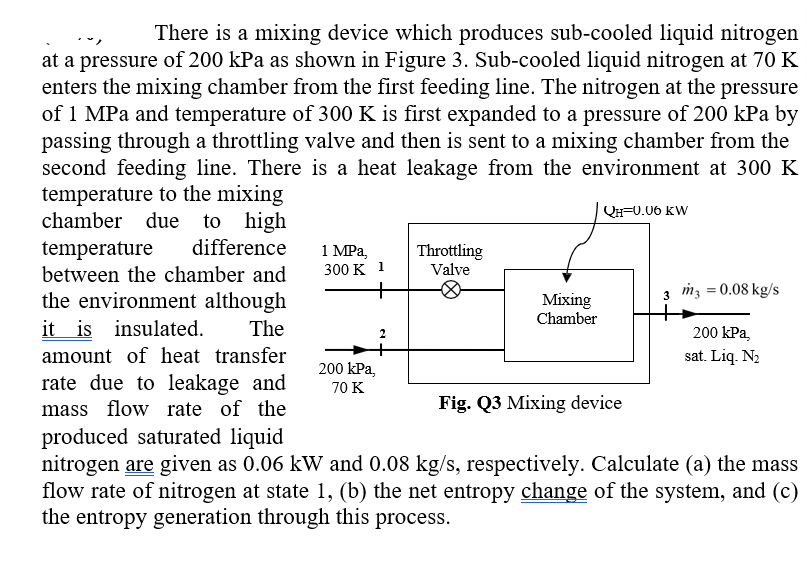
Question: There is a mixing device which produces sub - cooled liquid nitrogen at a pressure of 2 0 0 kPa as shown in Figure 3
There is a mixing device which produces subcooled liquid nitrogen at a pressure of kPa as shown in Figure Subcooled liquid nitrogen at K enters the mixing chamber from the first feeding line. The nitrogen at the pressure of MPa and temperature of K is first expanded to a pressure of kPa by passing through a throttling valve and then is sent to a mixing chamber from the second feeding line. There is a heat leakage from the environment at K temperature to the mixing chamber due to high temperature difference between the chamber and the environment although it is insulated. The amount of heat transfer rate due to leakage and mass flow rate of the produced saturated liquid nitrogen are given as kW and mathrm~kgmathrms respectively. Calculate a the mass flow rate of nitrogen at state b the net entropy change of the system, and c the entropy generation through this process.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


