Question: there is not information to be added to this question, respectfully if you do not know how to complete it please dont mark my questions
there is not information to be added to this question, respectfully if you do not know how to complete it please dont mark my questions as needing mote info. all the info needed is in the picture provided.. 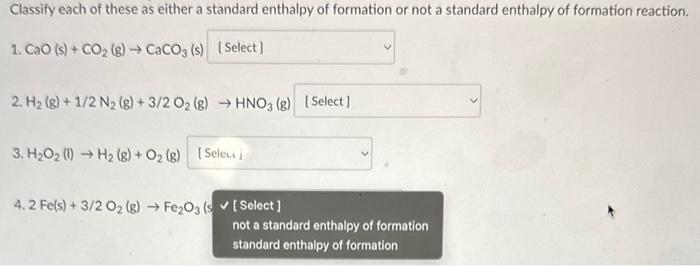

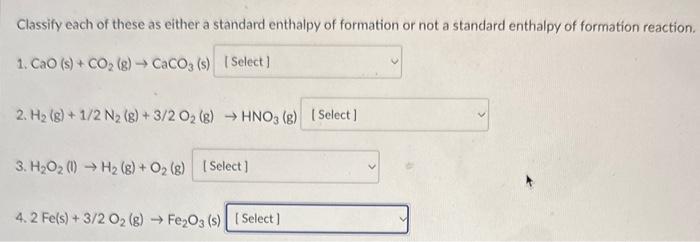
Classify each of these as either a standard enthalpy of formation or not a standard enthalpy of formation reaction. 1. CaO (s) +CO2(g)CaCO3 (s) 2. H2(g)+1/2N2(g)+3/2O2(g)HNO3(g) 3. H2O2(l)H2(g)+O2(g) 4. 2Fe(s)+3/2O2(g)Fe2O3(s) Classify each of these as either a standard enthalpy of formation or not a standard enthalpy of formation reaction. 1. CaO(s)+CO2(g)CaCO3(s) 2. H2(g)+1/2N2(g)+3/2O2(g)HNO3(g) 3. H2O2(l)H2(g)+O2(g) 4. 2Fe(s)+3/2O2(g)Fe2O3 (s
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


