Question: There is one question in here. Can you please be quick. 3:) Carbon monoxide (CO) and hydrogen (H2) catalytically to produce methanol (CH3OH) he reacts.

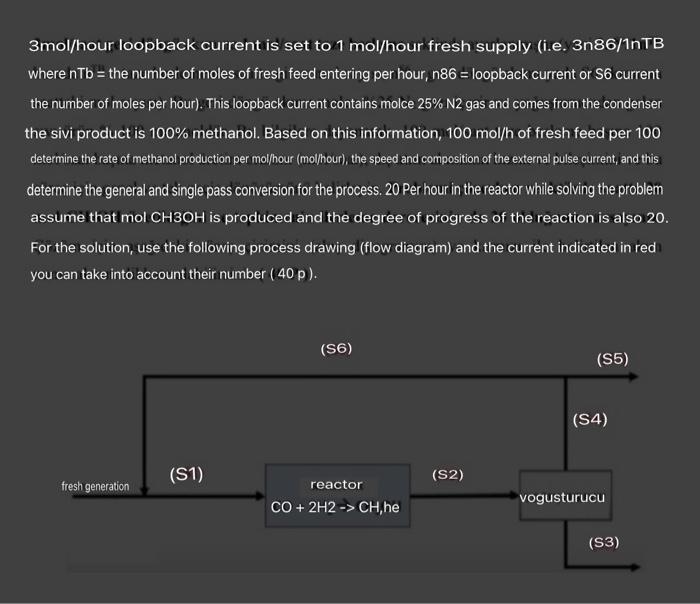
3:) Carbon monoxide (CO) and hydrogen (H2) catalytically to produce methanol (CH3OH) he reacts. Fresh feed composition molce 30%CO,H2 in stoichiometric ratio (i.e. 60\%) and it contains 10%N2 (inert (nonreactive)). A condenser has not reacted it separates gases and inertias from the liquid product CHOH. Part of the gas stream is the main supply to the reactor it is returned to the line and the rest leaves the process as an external pulse current. Loopback rate, 3mol/ hour loopback current is set to 1mol/hour fresh supply (i.e. 3n86/1nTB where nTb= the number of moles of fresh feed entering per hour, n86= loopback current or S6 current the number of moles per hour). This loopback current contains molce 25% N2 gas and comes from the condenser the sivi product is 100% methanol. Based on this information, 100mol/h of fresh feed per 100 determine the rate of methanol production per mol/hour (molhour), the speed and composition of the external pulse current, and this determine the general and single pass conversion for the process. 20 Per hour in the reactor while solving the problem assume that molCH3OH is produced and the degree of progress of the reaction is also 20. For the solution, use the following process drawing (flow diagram) and the current indicated in redi you can take into account their number (40p)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


