Question: Thermochemistry During a combustion process burning Ethene (C2H4) with 200% theoretical air. Assume complete combustion and a total pressure of 120 KPa. Determine: a) Air-fuel
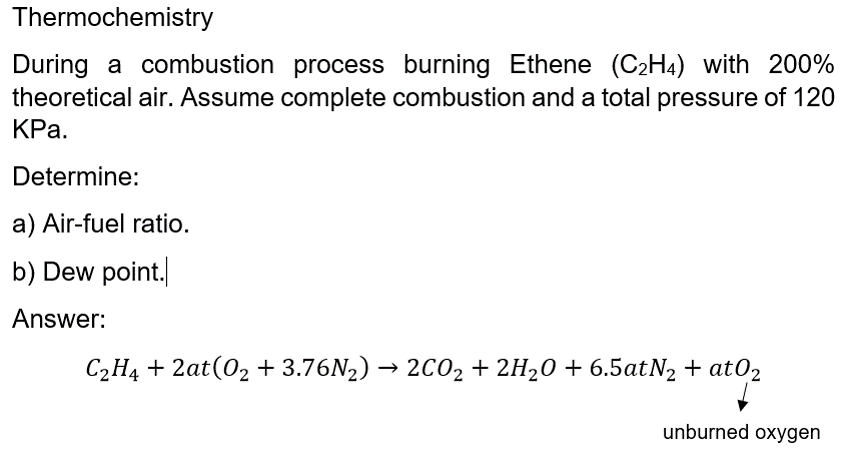
Thermochemistry During a combustion process burning Ethene (C2H4) with 200% theoretical air. Assume complete combustion and a total pressure of 120 KPa. Determine: a) Air-fuel ratio. b) Dew point. Answer: C2H4 + 2at(02 +3.76N2) 2002 + 2H20 + 6.5atN2 + atO2 unburned oxygen
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


