Question: THERMOCHEMISTRY SHOW SOLUTION AND BOX THE FINAL ANSWER. ROUND OFF THE ANSWER TO 3 DECIMAL FIGURES. 4. When 7.474 g of NaCl is dissolved in
THERMOCHEMISTRY
SHOW SOLUTION AND BOX THE FINAL ANSWER. ROUND OFF THE ANSWER TO 3 DECIMAL FIGURES.

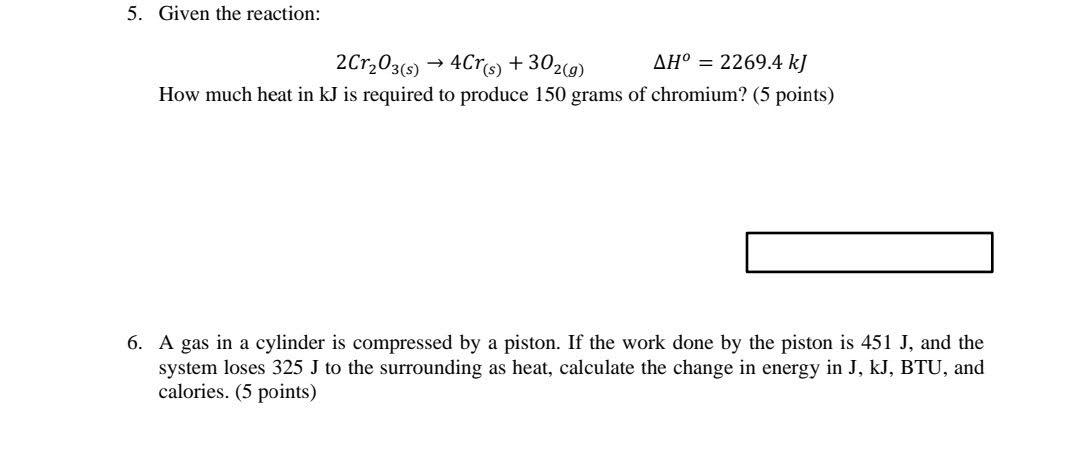
4. When 7.474 g of NaCl is dissolved in 120 grams of water at 22C in a coffee-cup calorimeter, 500J of heat are absorbed by the solution. (Hint: If heat is absorbed by the solution, the reaction produces heat for the solution to absorb.) (8 points) a. What is the final temperature in "C? b. What is the heat of the solution in kJ/mol? 5. Given the reaction: 2Cr2O3(8) 4C76) + 302(9) AH = 2269.4 kJ How much heat in kJ is required to produce 150 grams of chromium? (5 points) 6. A gas in a cylinder is compressed by a piston. If the work done by the piston is 451 J, and the system loses 325 J to the surrounding as heat, calculate the change in energy in J, KJ, BTU, and calories. (5 points)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


