Question: Thermodynamics (Open Book, Ruler and a Calculator) 1. a. It was observed that when the atom percent of Cu was greater than or equal
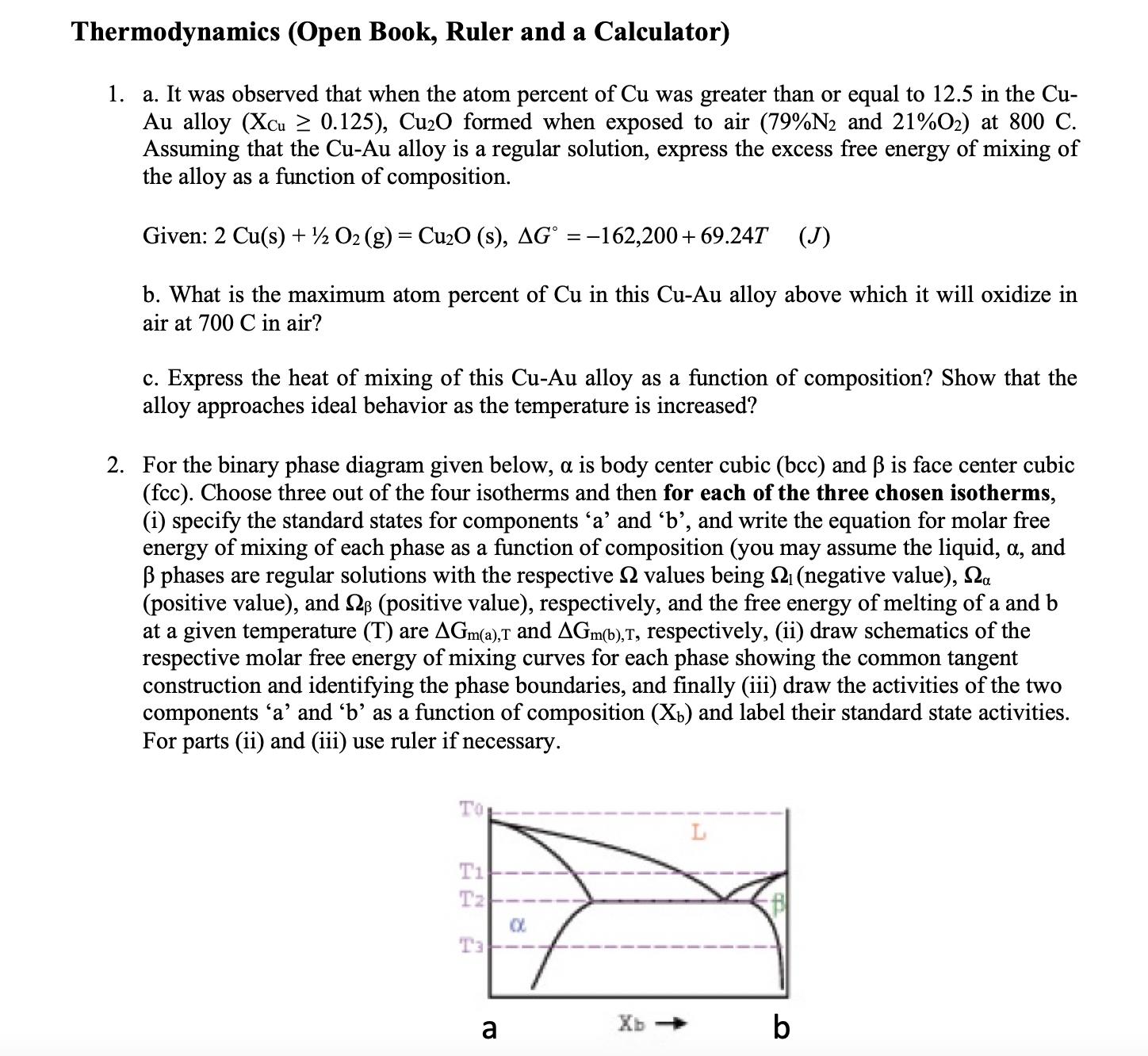
Thermodynamics (Open Book, Ruler and a Calculator) 1. a. It was observed that when the atom percent of Cu was greater than or equal to 12.5 in the Cu- Au alloy (Xcu 0.125), Cu2O formed when exposed to air (79%2 and 21%02) at 800 C. Assuming that the Cu-Au alloy is a regular solution, express the excess free energy of mixing of the alloy as a function of composition. Given: 2 Cu(s) + O2 (g) = Cu2O (s), AG = -162,200+ 69.24T (J) b. What is the maximum atom percent of Cu in this Cu-Au alloy above which it will oxidize in air at 700 C in air? c. Express the heat of mixing of this Cu-Au alloy as a function of composition? Show that the alloy approaches ideal behavior as the temperature is increased? 2. For the binary phase diagram given below, a is body center cubic (bcc) and is face center cubic (fcc). Choose three out of the four isotherms and then for each of the three chosen isotherms, (i) specify the standard states for components 'a' and 'b', and write the equation for molar free energy of mixing of each phase as a function of composition (you may assume the liquid, , and phases are regular solutions with the respective Q values being (negative value), a (positive value), and 2 (positive value), respectively, and the free energy of melting of a and b at a given temperature (T) are AGm(a),T and AGm(b),T, respectively, (ii) draw schematics of the respective molar free energy of mixing curves for each phase showing the common tangent construction and identifying the phase boundaries, and finally (iii) draw the activities of the two components 'a' and 'b' as a function of composition (Xb) and label their standard state activities. For parts (ii) and (iii) use ruler if necessary. Top 22 T1 Tz Ta Pe a Xb b
Step by Step Solution
There are 3 Steps involved in it
1 CuAu Alloy Thermodynamics Problem a Free Energy of Mixing of the Alloy as a Function of Composition The CuAu alloy is described as a regular solution and we need to express the excess free energy of ... View full answer

Get step-by-step solutions from verified subject matter experts


