Question: Thermodynamics problem. Please explain and show all steps. Thank you The following reversible dimerization reaction occurs in a cell of volume V : 2AB In
Thermodynamics problem. Please explain and show all steps. Thank you
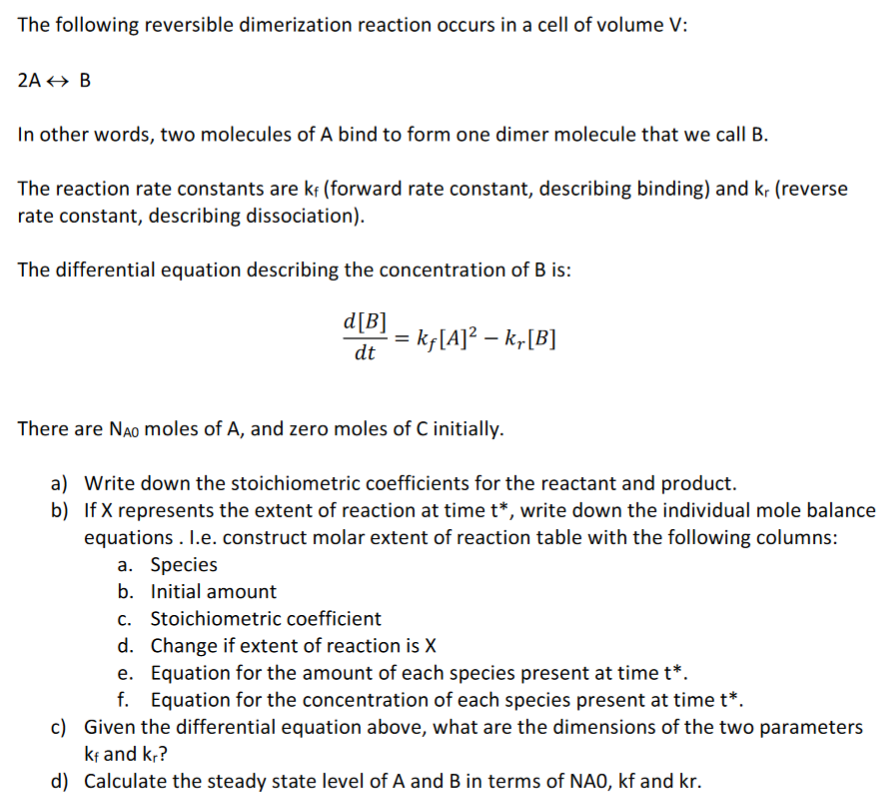
The following reversible dimerization reaction occurs in a cell of volume V : 2AB In other words, two molecules of A bind to form one dimer molecule that we call B. The reaction rate constants are kf (forward rate constant, describing binding) and kr (reverse rate constant, describing dissociation). The differential equation describing the concentration of B is: dtd[B]=kf[A]2kr[B] There are NA0 moles of A, and zero moles of C initially. a) Write down the stoichiometric coefficients for the reactant and product. b) If X represents the extent of reaction at time t, write down the individual mole balance equations . I.e. construct molar extent of reaction table with the following columns: a. Species b. Initial amount c. Stoichiometric coefficient d. Change if extent of reaction is X e. Equation for the amount of each species present at time t *. f. Equation for the concentration of each species present at time t. c) Given the differential equation above, what are the dimensions of the two parameters kf and kr ? d) Calculate the steady state level of A and B in terms of NA0,kf and kr
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


