Question: Thermodynamics Question 2: 2. (a) State the first and second laws of thermodynamics. Use one example for each law to describe how they are applied
Thermodynamics Question 2:
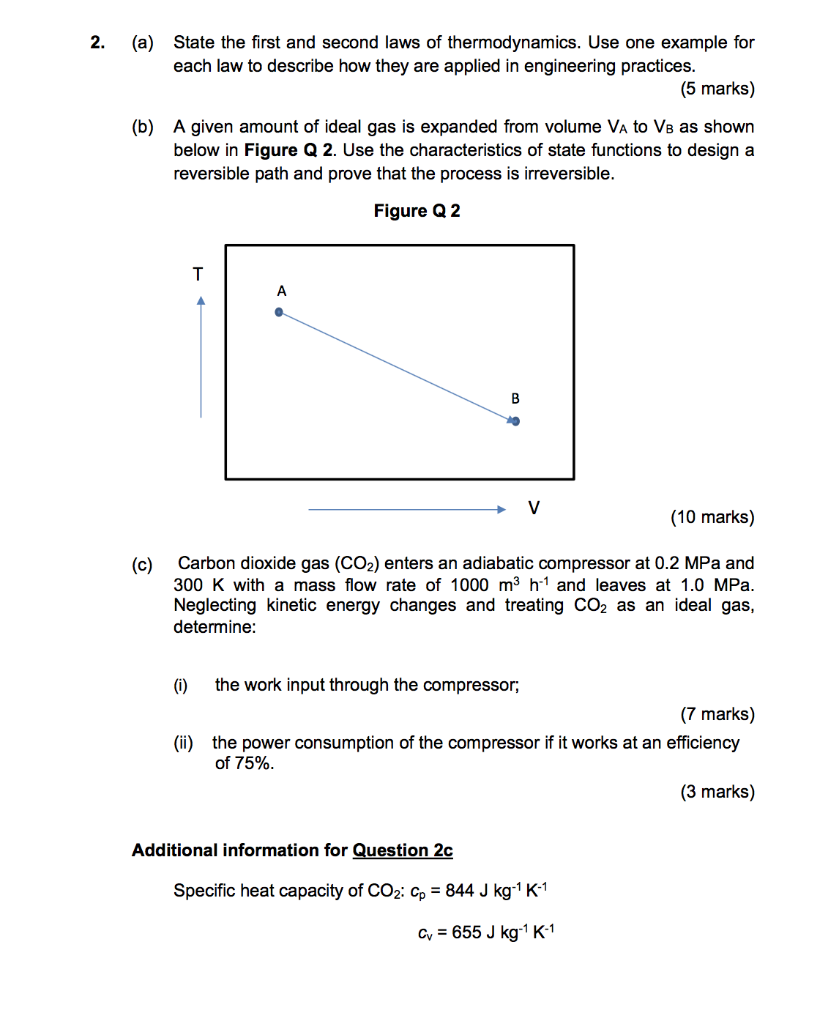
2. (a) State the first and second laws of thermodynamics. Use one example for each law to describe how they are applied in engineering practices. (5 marks) (b) A given amount of ideal gas is expanded from volume VA to VB as shown below in Figure Q 2. Use the characteristics of state functions to design a reversible path and prove that the process is irreversible. Figure Q2 T A B V (10 marks) (c) Carbon dioxide gas (CO2) enters an adiabatic compressor at 0.2 MPa and 300 K with a mass flow rate of 1000 m3 h-1 and leaves at 1.0 MPa. Neglecting kinetic energy changes and treating CO2 as an ideal gas, determine: (0) the work input through the compressor, (7 marks) (ii) the power consumption of the compressor if it works at an efficiency of 75% (3 marks) Additional information for Question 2c Specific heat capacity of CO2: Cp = 844 J kg-1 K-1 Cv = 655 J kg K-1 2. (a) State the first and second laws of thermodynamics. Use one example for each law to describe how they are applied in engineering practices. (5 marks) (b) A given amount of ideal gas is expanded from volume VA to VB as shown below in Figure Q 2. Use the characteristics of state functions to design a reversible path and prove that the process is irreversible. Figure Q2 T A B V (10 marks) (c) Carbon dioxide gas (CO2) enters an adiabatic compressor at 0.2 MPa and 300 K with a mass flow rate of 1000 m3 h-1 and leaves at 1.0 MPa. Neglecting kinetic energy changes and treating CO2 as an ideal gas, determine: (0) the work input through the compressor, (7 marks) (ii) the power consumption of the compressor if it works at an efficiency of 75% (3 marks) Additional information for Question 2c Specific heat capacity of CO2: Cp = 844 J kg-1 K-1 Cv = 655 J kg K-1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


