Question: these answers are NOT correct. please do not answer if you dont know what you are doing. if you repost the other answers here on
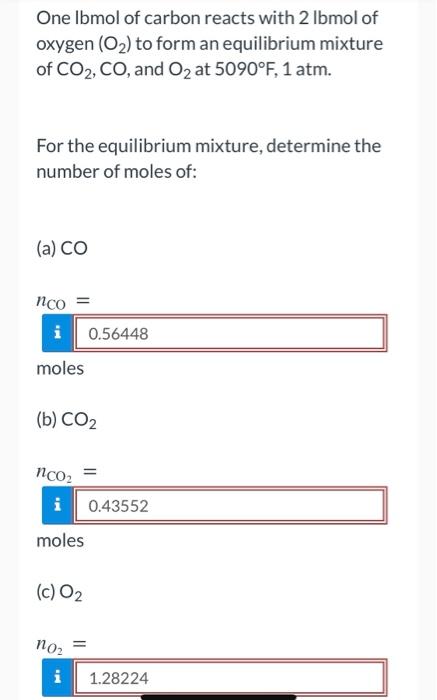
One lbmol of carbon reacts with 2 lbmol of oxygen (O2) to form an equilibrium mixture of CO2, CO, and O2 at 5090F, 1 atm. For the equilibrium mixture, determine the number of moles of: (a) CO nco = i 0.56448 moles (b) CO2 nCO2 = i 0.43552 moles (c) O2 noz i 1.28224
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


