Question: These are the original assumptions and data, please help me find out the second part Assume that only the equilibrium potential for cathodic reaction is
These are the original assumptions and data, please help me find out the second part
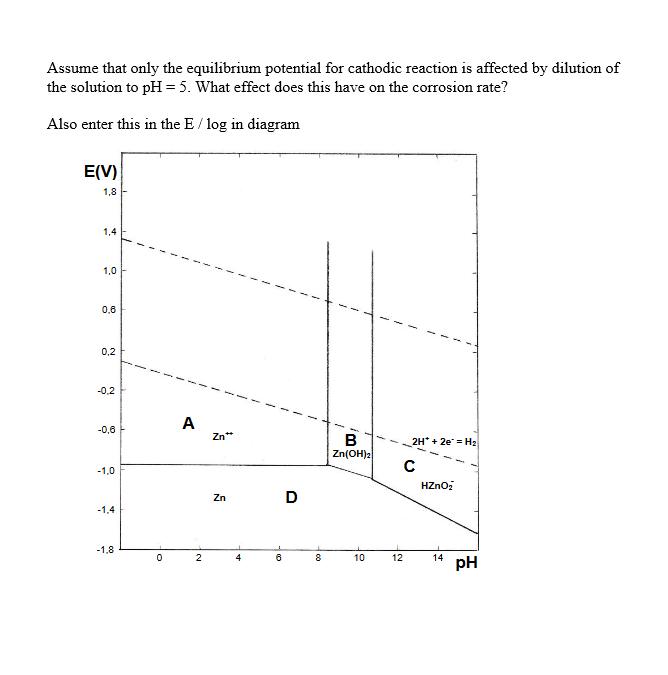
Assume that only the equilibrium potential for cathodic reaction is affected by dilution of the solution to pH = 5. What effect does this have on the corrosion rate? Also enter this in the E / log in diagram E(V) 1,8 1,4 1,0 0.8 0,2 -0.2 A -0.6 Zn** 1 B Zn(OH)2 2H* + 2e = H2 -1,0 HZnO Zn D -1.4 -1,8 0 2 4 8 00 10 12 14 pH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


