Question: These questions are meant to be coded in python! Data @ 32.86 kPa . Use nonlinear regression to determine the parameters in the model equations
These questions are meant to be coded in python!
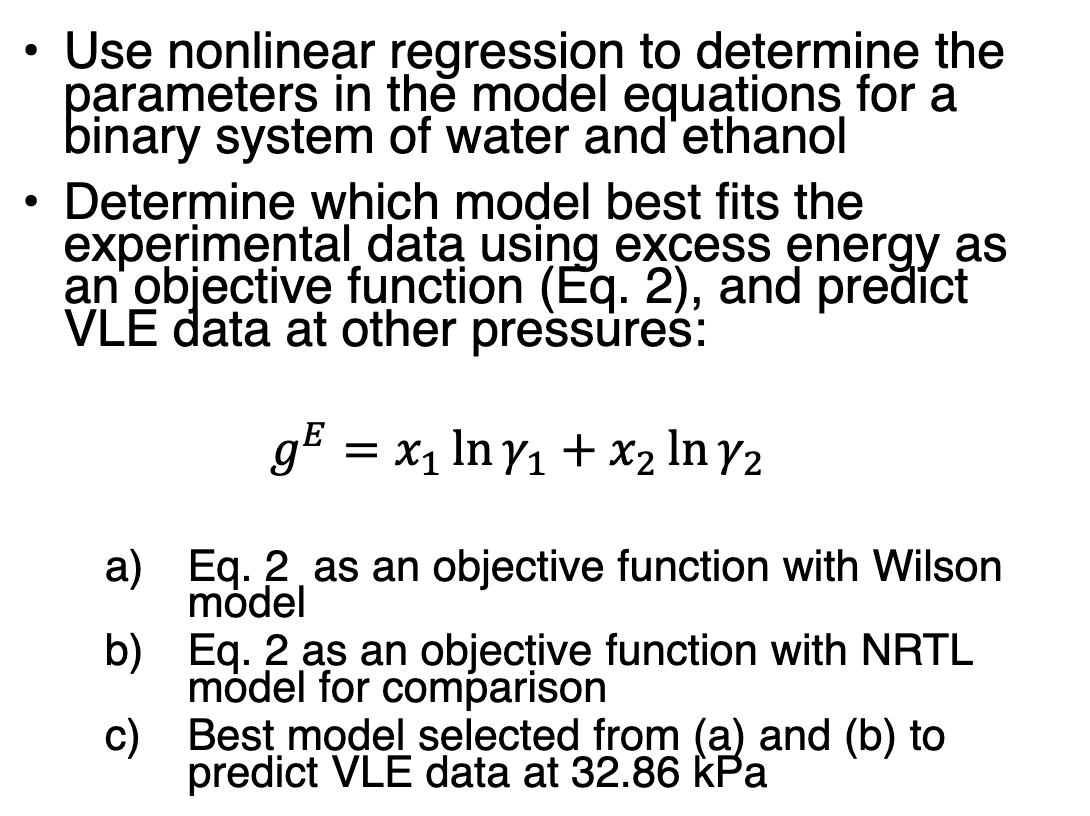
Data @ 32.86 kPa
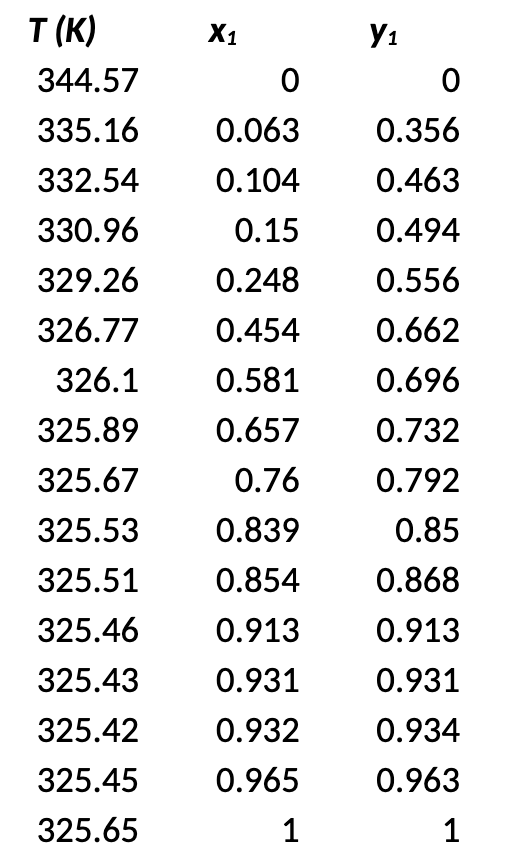
. Use nonlinear regression to determine the parameters in the model equations for a binary system of water and ethanol Determine which model best fits the experimental data using excess energy as an objective function (Eq. 2), and predict VLE data at other pressures: . ) g = x1 In Y1 + x2 In 72 X1 a) Eq. 2. as an objective function with Wilson model b) Eq. 2 as an objective function with NRTL model for comparison c) Best model selected from (a) and (b) to predict VLE data at 32.86 kPa X1 0 0.063 0.104 0.15 0.248 T(K) 344.57 335.16 332.54 330.96 329.26 326.77 326.1 325.89 325.67 325.53 325.51 325.46 325.43 325.42 325.45 325.65 0.454 0.581 0.657 0.76 0.839 Y1 0 0.356 0.463 0.494 0.556 0.662 0.696 0.732 0.792 0.85 0.868 0.913 0.931 0.934 0.963 0.854 0.913 0.931 0.932 0.965 1 1 . Use nonlinear regression to determine the parameters in the model equations for a binary system of water and ethanol Determine which model best fits the experimental data using excess energy as an objective function (Eq. 2), and predict VLE data at other pressures: . ) g = x1 In Y1 + x2 In 72 X1 a) Eq. 2. as an objective function with Wilson model b) Eq. 2 as an objective function with NRTL model for comparison c) Best model selected from (a) and (b) to predict VLE data at 32.86 kPa X1 0 0.063 0.104 0.15 0.248 T(K) 344.57 335.16 332.54 330.96 329.26 326.77 326.1 325.89 325.67 325.53 325.51 325.46 325.43 325.42 325.45 325.65 0.454 0.581 0.657 0.76 0.839 Y1 0 0.356 0.463 0.494 0.556 0.662 0.696 0.732 0.792 0.85 0.868 0.913 0.931 0.934 0.963 0.854 0.913 0.931 0.932 0.965 1 1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


