Question: This assignment is about Reactive Sputtering, but it utilizes concepts from the entire last half of the class. Even if you missed the one lecture

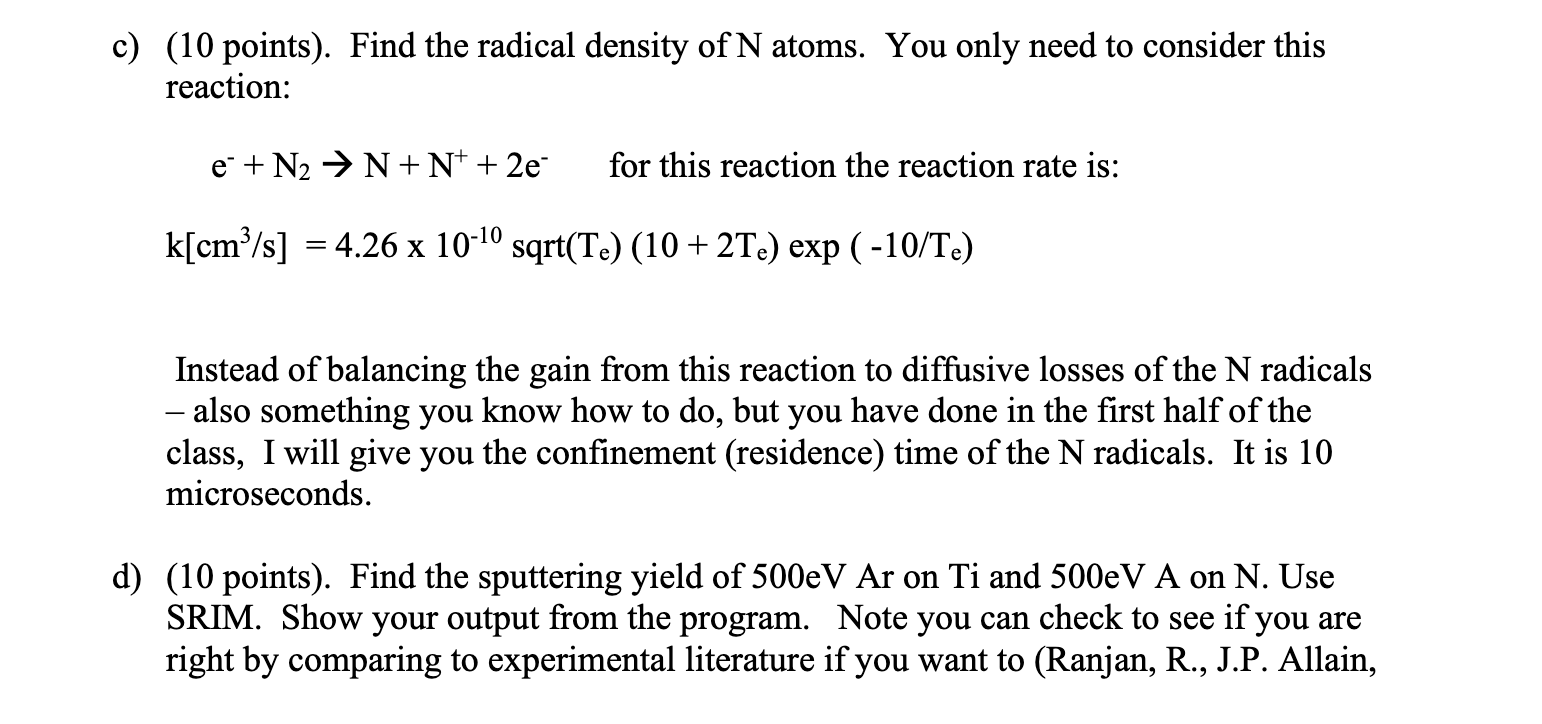
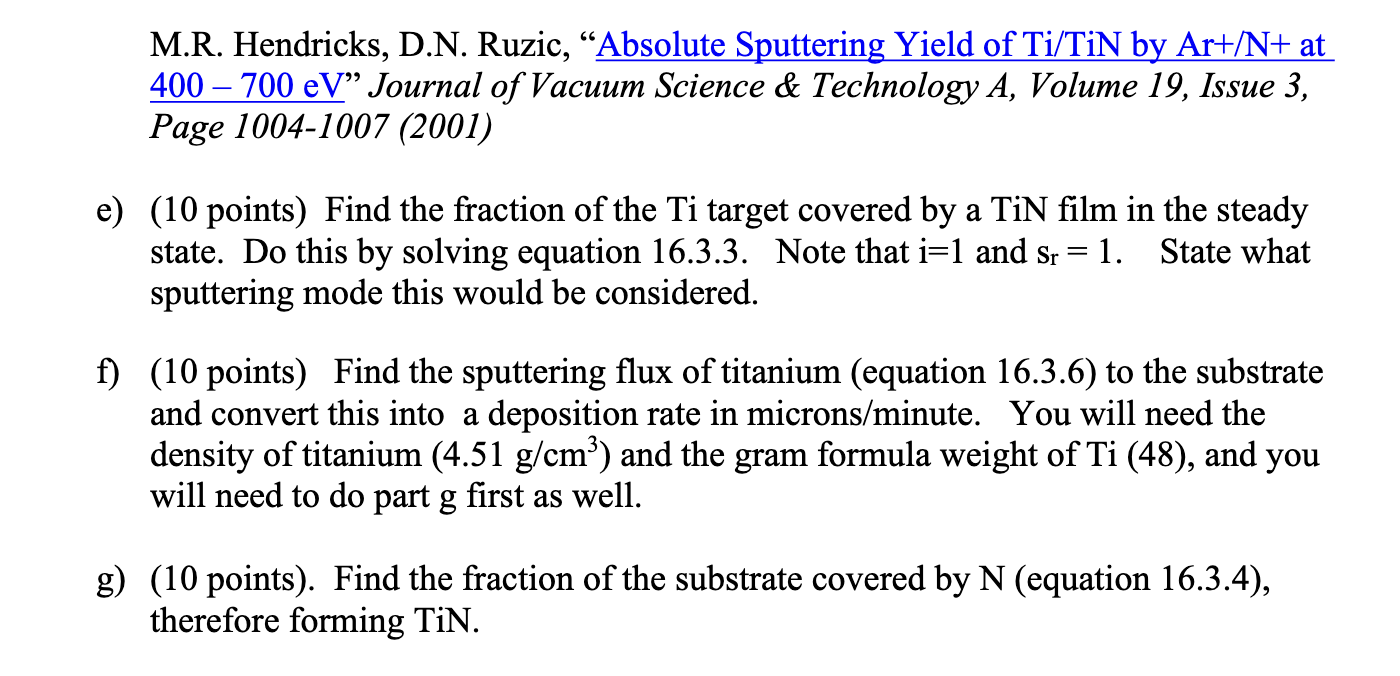

This assignment is about Reactive Sputtering, but it utilizes concepts from the entire last half of the class. Even if you missed the one lecture I did on this exact topic, look at pp. 632 -634 in L\&L. It has some of the equations you need. You have a PVD magnetron sputtering system with a Ti target and you want to create titanium-nitride (TiN) on your substrate. The area of the sputtered part of the target (the racetrack) is 5cm2. The area of the substrate you are covering is 50cm2. The DC voltage on the target is -500 Volts. 16 Watts of power is being consumed. The system is in a metal vacuum chamber that is grounded, so you can assume the plasma potential is 0 . The plasma does not extend to the substrate, so there is no sputtering of it from the plasma. You can also assume that all sputtered atoms that leave the target actually make it to the substrate. The pressure in the device is 50mTorr total. The gas mix is 99%Ar and 1%N2. Your overall goal is to find the deposition rate of TiN on the substrate and to ensure that the substrate ends up with a ratio of 1 Ti to 1N in the film. You have learned all the tools to do this, which is pretty cool. I will break down the problem into parts for you. a) (no points) The first thing to do would be to balance ionization to loss and determine an electron temperature. You have done that before and I will just give you the answer. The electron temperature is 4eV. b) (10 points). Find the bulk plasma density. Here are some hints: You can find the ion current to the target from knowing the power and voltage. You can then convert this into a flux. Since flux is conserved through the sheath and pre-sheath, you can conveniently calculate it at one point, and back out the value of density in the bulk. c) (10 points). Find the radical density of N atoms. You only need to consider this reaction: e+N2N+N++2e for this reaction the reaction rate is: k[cm3/s]=4.261010sqrt(Te)(10+2Te)exp(10/Te) Instead of balancing the gain from this reaction to diffusive losses of the N radicals - also something you know how to do, but you have done in the first half of the class, I will give you the confinement (residence) time of the N radicals. It is 10 microseconds. d) (10 points). Find the sputtering yield of 500eVAr on Ti and 500eVA on N. Use SRIM. Show your output from the program. Note you can check to see if you are right by comparing to experimental literature if you want to (Ranjan, R., J.P. Allain, M.R. Hendricks, D.N. Ruzic, "Absolute Sputtering Yield of Ti/TiN by Ar+/N+ at 400700eV " Journal of Vacuum Science \& Technology A, Volume 19, Issue 3, Page 1004-1007 (2001) e) (10 points) Find the fraction of the Ti target covered by a TiN film in the steady state. Do this by solving equation 16.3.3. Note that i=1 and sr=1. State what sputtering mode this would be considered. f) (10 points) Find the sputtering flux of titanium (equation 16.3.6) to the substrate and convert this into a deposition rate in microns/minute. You will need the density of titanium (4.51g/cm3) and the gram formula weight of Ti(48), and you will need to do part g first as well. g) (10 points). Find the fraction of the substrate covered by N (equation 16.3.4), therefore forming TiN. You want this number, the fraction of the substrate covered by N to be 1 , and if I did the problem correct it will be less than one using the input numbers given. Now the fun part. h) (40 points) What do you need to do in terms of altering items under your control (power, voltage, pressure, gas ratio) to get this number closer to one? Try to find realistic conditions to make this number go up ideally to 1 , and then show the results that get for that case, while still having a "decent" deposition rate. Show all the numbers for parts b), c), e), f) and g) and comment on the solution. As always, show all your work and list all your assumptions
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


