Question: This exercise will take us through the process of modeling glucose solubility in ethanol + water mixtures at 3 5 C . In some cases,
This exercise will take us through the process of modeling glucose solubility in ethanol
water mixtures at In some cases, limited data are available. The heat of fusion of
glucose is not available.
Goal: Predict glucose solubility in ternary mixtures from binary measurements.
Molecular weights are: Glucose EtOH Water
Method: We will use the Margules because it is simple and the system is isothermal. The
equilibrium relation is
I. Binary Glucose Water
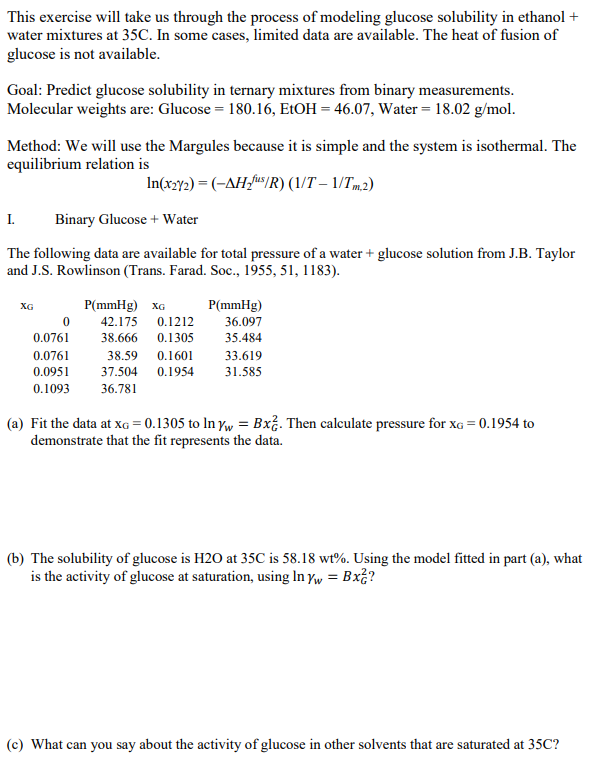
The following data are available for total pressure of a water glucose solution from JB Taylor
and JS Rowlinson Trans Farad. Soc.,
a Fit the data at to Then calculate pressure for to
demonstrate that the fit represents the data.
b The solubility of glucose is at is Using the model fitted in part a what
is the activity of glucose at saturation, using
c What can you say about the activity of glucose in other solvents that are saturated at This exercise will take us through the process of modeling glucose solubility in ethanol water mixtures at C In some cases, limited data are available. The heat of fusion of glucose is not available. Goal: Predict glucose solubility in ternary mixtures from binary measurements. Molecular weights are: Glucose EtOH Water gmol Method: We will use the Margules because it is simple and the system is isothermal. The equilibrium relation is lnxgamma Delta HfusRTTm I. Binary Glucose Water The following data are available for total pressure of a water glucose solution from JB Taylor and JS Rowlinson Trans Farad. Soc., xGPmmHgxGPmmHg
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


