Question: This for a lab. I will be doing the models but I'm struggling to understand the content. I need help filling out the number of
This for a lab. I will be doing the models but I'm struggling to understand the content. I need help filling out the number of valence electrons, drawing the Lewis Structure, and possibly some help on the questions listed at the bottom of the page. I will complete the rest. Thank you!!!
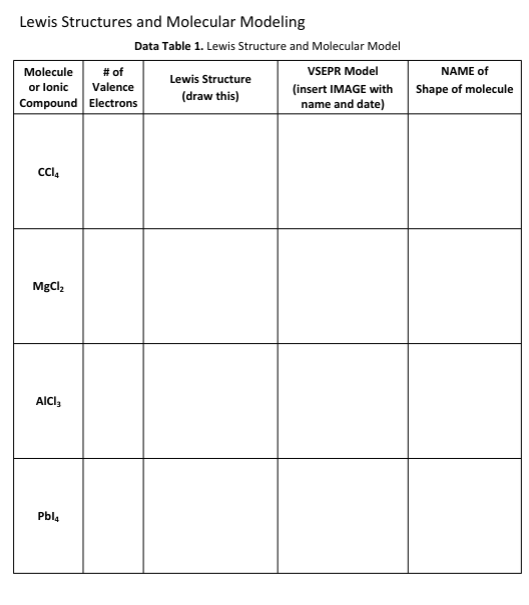
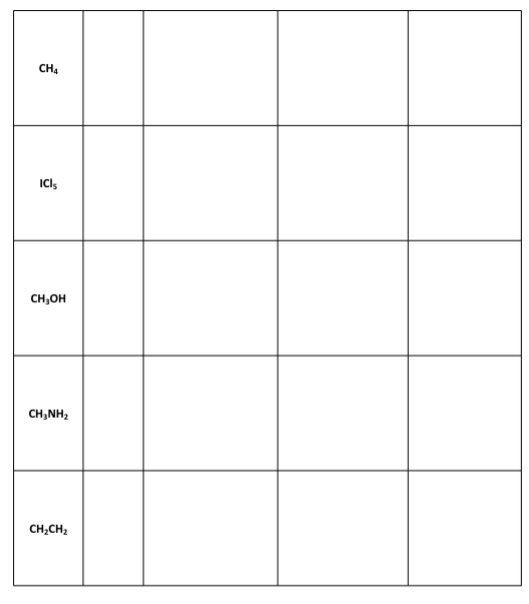
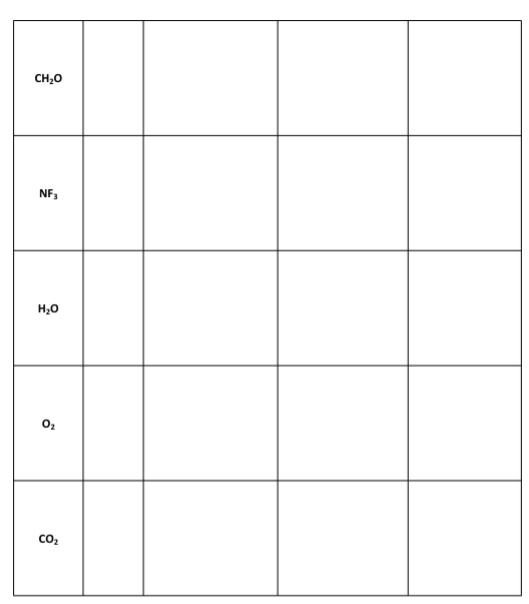
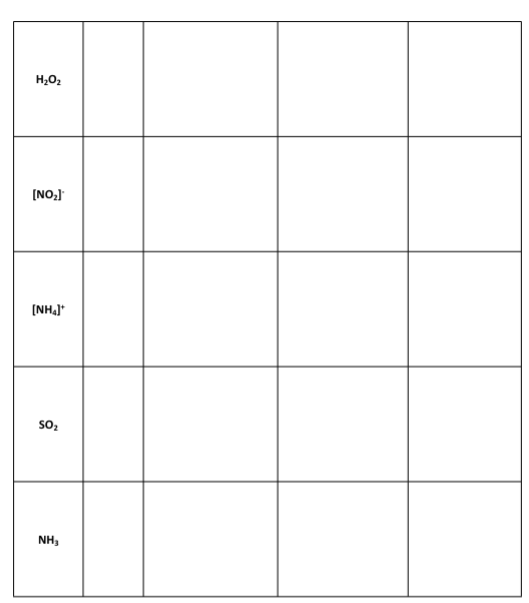
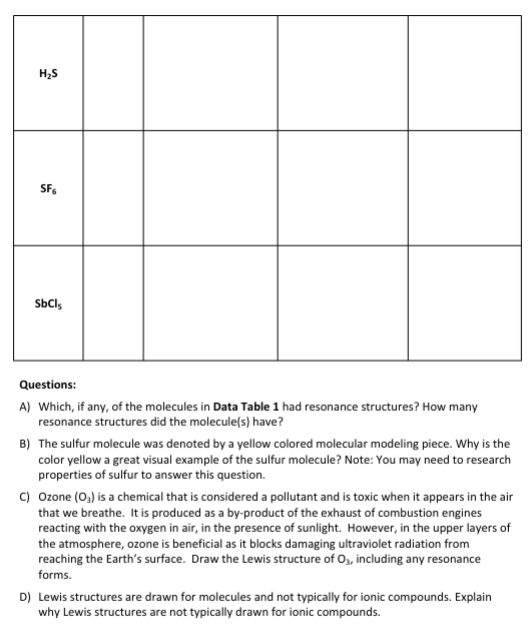
1r1 Questions: A) Which, if any, of the molecules in Data Table 1 had resonance structures? How many resonance structures did the molecule(s) have? B) The sulfur molecule was denoted by a yellow colored molecular modeling piece. Why is the color yellow a great visual example of the sulfur molecule? Note: You may need to research properties of sulfur to answer this question. C) Ozone (O3) is a chemical that is considered a pollutant and is toxic when it appears in the air that we breathe. It is produced as a by-product of the exhaust of combustion engines reacting with the oxygen in air, in the presence of sunlight. However, in the upper layers of the atmosphere, ozone is beneficial as it blocks damaging ultraviolet radiation from reaching the Earth's surface. Draw the Lewis structure of O3, including any resonance forms. D) Lewis structures are drawn for molecules and not typically for ionic compounds. Explain why Lewis structures are not typically drawn for ionic compounds
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


