Question: This has to do with voltaic electrochemical cells Post Lab Questions Answer the questions found at the end of the procedure document. Explain your reasoning

 This has to do with voltaic electrochemical cells
This has to do with voltaic electrochemical cells
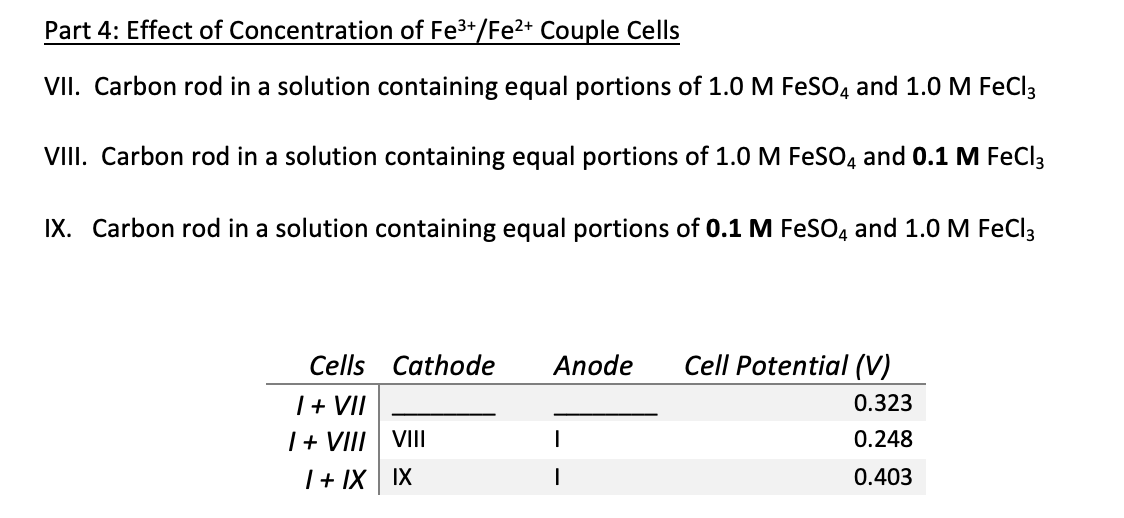
Post Lab Questions Answer the questions found at the end of the procedure document. Explain your reasoning completely. You do not need to rewrite the questions, but your TA must be able to tell which part of which question you are answering with any given statement. 1. Using Sample Question 1 from the Pre-Lab Assignment as a guide, draw the cell based on Compartments I and VII from Part 4 of the lab. Label all key components and illustrate the flow of electrons as well as ions through the salt bridge. a. If Compartment I was replaced by Compartment VIII, what type of cell would this become? b. Which compartment would be the anode and which would be the cathode? C. What material could be used for the electrode in Compartment VIII? 2. Typically, in a cell drawing the anode is shown on the left and the cathode on the right. Indicate the direction of flow for each of the following in a newly drawn cell where the anode was placed on the right and the cathode on the left. a. The flow of electrons b. The flow of cations through the salt bridge C. The flow of anions through the salt bridge What change in the potential shown on the voltmeter would be expected in a cell with the anode on the right and the cathode on the left? Part 4: Effect of Concentration of Fe3+/Fe2+ Couple Cells VII. Carbon rod in a solution containing equal portions of 1.0 M FeSO4 and 1.0 M FeCl3 VIII. Carbon rod in a solution containing equal portions of 1.0 M FeSO4 and 0.1 M FeCl3 IX. Carbon rod in a solution containing equal portions of 0.1 M FeSO4 and 1.0 M FeCl3 Anode Cells Cathode 1 + VII I + VII VIII | + IX IX Cell Potential (V) 0.323 0.248 0.403 Post Lab Questions Answer the questions found at the end of the procedure document. Explain your reasoning completely. You do not need to rewrite the questions, but your TA must be able to tell which part of which question you are answering with any given statement. 1. Using Sample Question 1 from the Pre-Lab Assignment as a guide, draw the cell based on Compartments I and VII from Part 4 of the lab. Label all key components and illustrate the flow of electrons as well as ions through the salt bridge. a. If Compartment I was replaced by Compartment VIII, what type of cell would this become? b. Which compartment would be the anode and which would be the cathode? C. What material could be used for the electrode in Compartment VIII? 2. Typically, in a cell drawing the anode is shown on the left and the cathode on the right. Indicate the direction of flow for each of the following in a newly drawn cell where the anode was placed on the right and the cathode on the left. a. The flow of electrons b. The flow of cations through the salt bridge C. The flow of anions through the salt bridge What change in the potential shown on the voltmeter would be expected in a cell with the anode on the right and the cathode on the left? Part 4: Effect of Concentration of Fe3+/Fe2+ Couple Cells VII. Carbon rod in a solution containing equal portions of 1.0 M FeSO4 and 1.0 M FeCl3 VIII. Carbon rod in a solution containing equal portions of 1.0 M FeSO4 and 0.1 M FeCl3 IX. Carbon rod in a solution containing equal portions of 0.1 M FeSO4 and 1.0 M FeCl3 Anode Cells Cathode 1 + VII I + VII VIII | + IX IX Cell Potential (V) 0.323 0.248 0.403
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


