Question: This is a Bronsted-Lowry acid-base reaction. Draw structures for all the products, showing any non-zero formal charges in each one. Make sure your reaction is
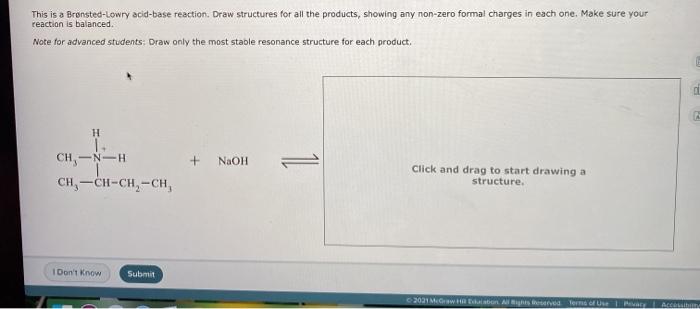
This is a Bronsted-Lowry acid-base reaction. Draw structures for all the products, showing any non-zero formal charges in each one. Make sure your reaction is balanced. Note for advanced students: Draw only the most stable resonance structure for each product. H CH-N-H. + NaOH 1 CH, -CH-CH-CH Click and drag to start drawing a structure. I Don't Know Submit 2001 M Habion All Righted
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


