Question: THIS IS A CHEMICAL ENGINEERING QUESTION. This is for the course Reaction Engineering ( this course concerns the design of reactors ) . Show all
THIS IS A CHEMICAL ENGINEERING QUESTION. This is for the course Reaction Engineering this course concerns the design of reactors Show all steps, the MATLAB CODE that needs to be used to solve this question and the graph generated. SOLVE CORRECTLY FOR THUMBS UP
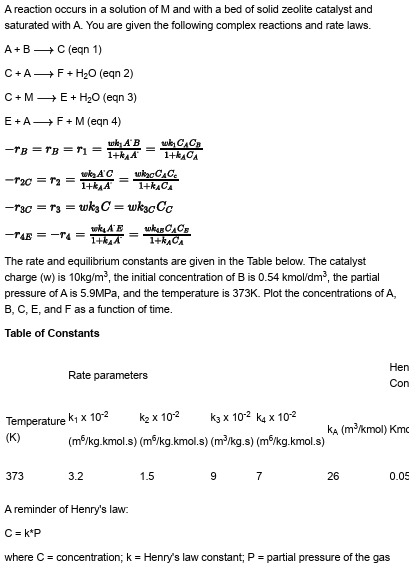
A reaction occurs in a solution of and with a bed of solid zeolite catalyst and saturated with You are given the following complex reactions and rate laws.
BlongrightarrowC
AlongrightarrowF
MlongrightarrowE
AlongrightarrowF
The rate and equilibrium constants are given in the Table below. The catalyst charge is the initial concentration of is kmo the partial pressure of is MPa, and the temperature is Plot the concentrations of and as a function of time.
Table of Constants
A reminder of Henry's law.
where concentration; Henry's law constant; partial pressure of the gas
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


