Question: this is a chemistry lab calculation question below is the volume of hcl used for titration use the information to find the initial quantity of




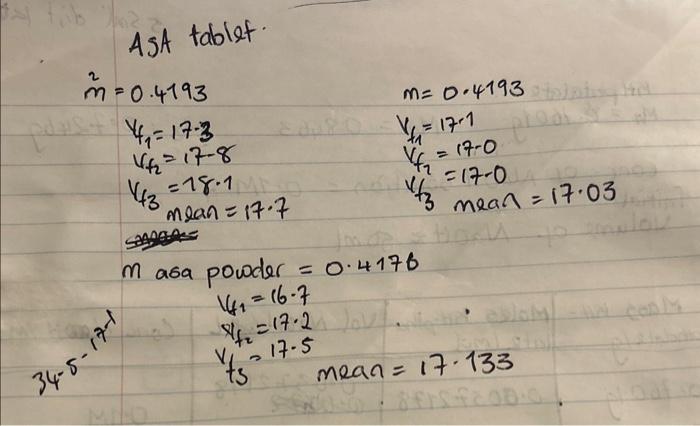
Preparation of 0.1M Hydrochloric Acid 1. Add 8.6mL of concentrated hydrochloric acid (11.6 M) to 500mL (use a measuring cylinder) of tap water in a glass storage bottle and make up to 1 litre by adding approximately 490mL water. Concentrated acid should always be added to water, never the other way around. 2. Mix well by inverting the sealed bottle several times. Standardization of 0.1M Hydrochloric Acid 1. Rinse the 25mL pipette 3 times with small portions of HCl. 2. Pipette 25mL of the hydrochloric acid into an erlenmeyer flask. 3. Add 23 drops of phenolphthalein to the solution in the erlenmeyer flask and titrate with standardized 0.1MNaOH as described above. 4. Kepeat the titration until 3 titrations differ by no more than 0.5%. 5. Calculate the concentration of the HCl. Preparation of Acetyl Salicylate Solution 1. Weigh with precision 3 aspirin tablets and place each tablet in a separate 100mL volumetric flask. (The flasks must be clean and dry. Remember not to dry the flasks in an oven.) 2. Label each flask clearly. 3. Add appravimately 75mL of 0.1MNaOH to each flask and shake well until the tablet is dissolved. A small quantity of white residue may not dissolve. 4. Fill the flask to the 100mL calibration mark with 0.1MNaOH. 5. Shake well to mix contents. Back titration of the excess NaOH 1. Empty the burette and wash well with tap water, then rinse with distilled water. 2. Rinse the burette with HCl before filling. 3. Use the same procedure as previously used with the NaOH. 4. Pipette 25mL of the acetyl salicylate solution into an erlenmeyer flask. If there is any residue on the bottom of the flask, be careful not to disturb it or suck any of it into the pipette. 5. Add 2 - 3Mrops of phenolphthalein and titrate with the hydrochloric acid until a faint pink colour persists. 6. Repeat the titration 2 more times. Your results should agree within 0.5%. 7. Calculate the initial quantity of NaOH (in 25mL ), the amount of NaOH in excess and determine the quantity of acetyl salicylic acid in each tablet. 8. Calculate the ASA as a \%wt. Analysis of aspirin powder 1. A number of aspirin tablets have been crushed and ground into a homogenous mixture. You will be given 1 portion of this powder to analyze for the concentration of aspirin. 2. Precisely weigh an amount of aspirin powder roughly equal to the weight of an aspirin tablet. 3. Analyze the ASA as described above. 4. Calculate the amount of ASA as a \%wt concentration. Calculate the concentration of ASA (\% wt) in 3 separate tablets using the data obtained above. This will give you data for each tablet, and along with the mass ASA per tablet, will allow you to calculate statistics for the tablet-to-tablet variation. Hand your data into the TA at the end of the lab. Solution concentrations (NaOH and HCl) should be as molarities, and ASA concentrations as \%weight. Ast tablef. m=0.4193vf1=173vf2=178vf3=18.17=17.0mean=17.7m=0.4193vf1=17.1vf1=17.0v3=mean=17.03 m asa powder =0.4176
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


