Question: This is a homework question! Let's break it down. Ke for the reaction F2(g)+Cl2(g)2FCl(g) equals 125 at a particular temperature. Suppose a system involving this
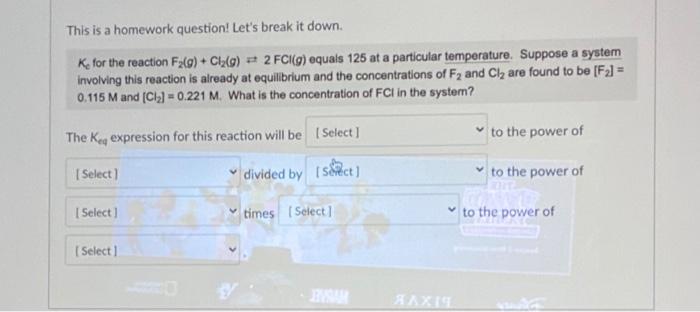
This is a homework question! Let's break it down. Ke for the reaction F2(g)+Cl2(g)2FCl(g) equals 125 at a particular temperature. Suppose a system involving this reaction is already at equilibrium and the concentrations of F2 and Cl2 are found to be [F2]= 0.115M and [Cl2]=0.221M. What is the concentration of FCl in the system? The Keq expression for this reaction will be to the power of divided by to the power of times to the power of
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


