Question: This is a problem from a material balances class. I have tried to solve this using a MatLab code, but have had little luck with
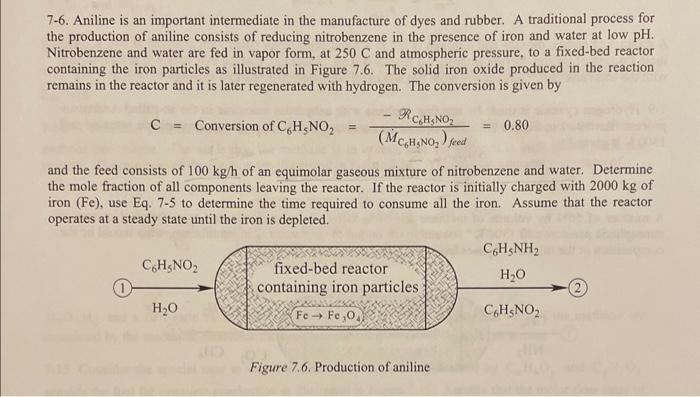
7-6. Aniline is an important intermediate in the manufacture of dyes and rubber. A traditional process for the production of aniline consists of reducing nitrobenzene in the presence of iron and water at low pH. Nitrobenzene and water are fed in vapor form, at 250C and atmospheric pressure, to a fixed-bed reactor containing the iron particles as illustrated in Figure 7.6. The solid iron oxide produced in the reaction remains in the reactor and it is later regenerated with hydrogen. The conversion is given by C=ConversionofC6H5NO2=(MC6H5NO2)cedRC6H5NO2=0.80 and the feed consists of 100kgh of an equimolar gaseous mixture of nitrobenzene and water. Determine the mole fraction of all components leaving the reactor. If the reactor is initially charged with 2000kg of iron ( Fe ), use Eq. 75 to determine the time required to consume all the iron. Assume that the reactor operates at a steady state until the iron is depleted. Figure 7.6. Production of aniline
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


